OXPHOS-Mediated Induction of NAD+ Promotes Complete Oxidation of Fatty Acids and Interdicts Non-Alcoholic Fatty Liver Disease
- PMID: 25933096
- PMCID: PMC4416931
- DOI: 10.1371/journal.pone.0125617
OXPHOS-Mediated Induction of NAD+ Promotes Complete Oxidation of Fatty Acids and Interdicts Non-Alcoholic Fatty Liver Disease
Abstract
OXPHOS is believed to play an important role in non-alcoholic fatty liver disease (NAFLD), however, precise mechanisms whereby OXPHOS influences lipid homeostasis are incompletely understood. We previously reported that ectopic expression of LRPPRC, a protein that increases cristae density and OXPHOS, promoted fatty acid oxidation in cultured primary hepatocytes. To determine the biological significance of that observation and define underlying mechanisms, we have ectopically expressed LRPPRC in mouse liver in the setting of NAFLD. Interestingly, ectopic expression of LRPPRC in mouse liver completely interdicted NAFLD, including inflammation. Consistent with mitigation of NAFLD, two markers of hepatic insulin resistance--ROS and PKCε activity--were both modestly reduced. As reported by others, improvement of NAFLD was associated with improved whole-body insulin sensitivity. Regarding hepatic lipid homeostasis, the ratio of NAD+ to NADH was dramatically increased in mouse liver replete with LRPPRC. Pharmacological activators and inhibitors of the cellular respiration respectively increased and decreased the [NAD+]/[NADH] ratio, indicating respiration-mediated control of the [NAD+]/[NADH] ratio. Supporting a prominent role for NAD+, increasing the concentration of NAD+ stimulated complete oxidation of fatty acids. Importantly, NAD+ rescued impaired fatty acid oxidation in hepatocytes deficient for either OXPHOS or SIRT3. These data are consistent with a model whereby augmented hepatic OXPHOS increases NAD+, which in turn promotes complete oxidation of fatty acids and protects against NAFLD.
Conflict of interest statement
Figures
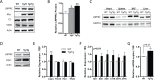


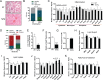
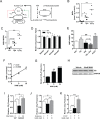

References
-
- Adams LA, Lymp JF, St SJ, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005. July;129(1):113–21. doi: S0016508505006943 [pii]. - PubMed
-
- Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000. January 18;132(2):112–7. doi: 200001180–00004 [pii]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

