Cost-effectiveness analysis of a vaccination program for the prevention of herpes zoster and post-herpetic neuralgia in adults aged 50 and over in Germany
- PMID: 25933182
- PMCID: PMC4514364
- DOI: 10.1080/21645515.2015.1011561
Cost-effectiveness analysis of a vaccination program for the prevention of herpes zoster and post-herpetic neuralgia in adults aged 50 and over in Germany
Abstract
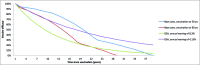
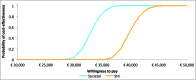
Herpes zoster (HZ; shingles) is a common viral disease that affects the nerves and surrounding skin causing a painful dermatomal rash and leading to debilitating complications such as, mainly, post-herpetic neuralgia (PHN). Currently, there is no effective treatment for HZ and PHN. The objective of this study was to assess the cost-effectiveness of a HZ vaccination program in Germany. An existing Markov Model was adapted to the German healthcare setting to compare a vaccination policy to no vaccination on a lifetime time-horizon, considering 2 scenarios: vaccinating people starting at the age of 50 or at the age of 60 years, from the perspective of the statutory health insurance (SHI) and the societal perspective. According to the perspective, vaccinating 20% of the 60+ German population resulted in 162,713 to 186,732 HZ and 31,657 to 35,793 PHN cases avoided. Corresponding incremental cost-effectiveness ratios (ICER) were 39,306 €/QALY from the SHI perspective and 37,417 €/QALY from a societal perspective. Results for the 50+ German population ranged from 336,468 to 394,575 HZ and from 48,637 to 56,087 PHN cases avoided from the societal perspective. Corresponding ICER were 39,782 €/QALY from a SHI perspective and 32,848 €/QALY from a societal perspective. Sensitivity analyses showed that results are mainly impacted by discount rates, utility values and use of alternative epidemiological data.The model indicated that a HZ vaccination policy in Germany leads to significant public health benefits and could be a cost-effective intervention. The results were robust and consistent with local and international existing literature.
Keywords: ASHIP, Association of Statutory Health Insurance Physicians; CEAC, Cost-effectiveness acceptability curves; CMI, Cell-mediated immunity; DSA, Deterministic sensitivity analysis; EBM, German uniform assessment standard (Einheitlicher Bewertungsmaßstab); EMA, European Medicines Agency; EQ-5D, EuroQoL; G-DRG, German Diagnosis Related Groups; GePaRD German Pharmacoepidemiological Research Database; Germany; HZ, Herpes zoster; ICER, Incremental cost-effectiveness ratio; IQWIG, German Institute for Quality and Efficiency in Health Care; NNV, Number needed to vaccinate; PHN, Post-herpetic neuralgia; PSA, Probabilistic sensitivity analysis; QALY, Quality-adjusted life year; SHI, Statutory health insurance; SPS, Shingles Prevention Study; STIKO, German Standing Committee on Immunisation; STPS, Short-Term Persistence Substudy; US, United States; VZV, Varizella zoster virus; YO, Years old; ZEST, Zostavax® Efficacy and Safety Trial; cost-effectiveness; herpes zoster; mBPI-SF Modified short form brief pain inventory; markov model; post-herpetic neuralgia; vaccination; zostavax.
Figures






Similar articles
-
Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany.BMC Health Serv Res. 2013 Sep 26;13:359. doi: 10.1186/1472-6963-13-359. BMC Health Serv Res. 2013. PMID: 24070414 Free PMC article.
-
Cost-Effectiveness of Herpes Zoster Vaccine for Persons Aged 50 Years.Ann Intern Med. 2015 Oct 6;163(7):489-97. doi: 10.7326/M15-0093. Ann Intern Med. 2015. PMID: 26344036
-
Cost-effectiveness of varicella vaccine against herpes zoster and post-herpetic neuralgia for elderly in Japan.Vaccine. 2017 May 31;35(24):3264-3271. doi: 10.1016/j.vaccine.2017.04.046. Epub 2017 May 4. Vaccine. 2017. PMID: 28479176
-
Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review.Vaccine. 2014 Mar 26;32(15):1645-53. doi: 10.1016/j.vaccine.2014.01.058. Epub 2014 Feb 15. Vaccine. 2014. PMID: 24534737 Review.
-
Cost-effectiveness of vaccination against herpes zoster.Hum Vaccin Immunother. 2014;10(7):2048-61. doi: 10.4161/hv.28670. Hum Vaccin Immunother. 2014. PMID: 25424815 Free PMC article. Review.
Cited by
-
Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development.Front Microbiol. 2022 Aug 10;13:932408. doi: 10.3389/fmicb.2022.932408. eCollection 2022. Front Microbiol. 2022. PMID: 36033843 Free PMC article. Review.
-
A Systematic Review of Methods for Estimating Productivity Losses due to Illness or Caregiving in Low- and Middle-Income Countries.Pharmacoeconomics. 2024 Aug;42(8):865-877. doi: 10.1007/s40273-024-01402-x. Epub 2024 Jun 14. Pharmacoeconomics. 2024. PMID: 38874846 Free PMC article.
-
Immune Dysregulation in Acute Herpes Zoster: Predictive Factors for Postherpetic Neuralgia.Med Sci Monit. 2025 Feb 8;31:e944688. doi: 10.12659/MSM.944688. Med Sci Monit. 2025. PMID: 39921244 Free PMC article.
-
Clinical and economic impact of herpes zoster vaccination in elderly in Italy.Hum Vaccin Immunother. 2017 Feb;13(2):405-411. doi: 10.1080/21645515.2017.1264832. Epub 2016 Dec 7. Hum Vaccin Immunother. 2017. PMID: 27925856 Free PMC article.
-
Herpes zoster vaccine: A health economic evaluation for Switzerland.Hum Vaccin Immunother. 2017 Jul 3;13(7):1495-1504. doi: 10.1080/21645515.2017.1308987. Epub 2017 May 8. Hum Vaccin Immunother. 2017. PMID: 28481678 Free PMC article.
References
-
- Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Microbiol 1993; 4:222–30; http://dx.doi.org/10.1097/00013542-199310000-00006 - DOI
-
- Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Phys 2000; 61:2437-48; PMID:10794584 - PubMed
-
- Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Prac 2002; 19:471-5; PMID:12356697; http://dx.doi.org/10.1093/fampra/19.5.471 - DOI - PubMed
-
- Oxman M, Levin M, Johnson G, Schmader K, Straus S, Gelb L, Arbeit R, Simberkoff M, Gershon A, Davis L. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271-84; PMID:15930418; http://dx.doi.org/10.1056/NEJMoa051016 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
