Factors Associated with Timing of Initiation of Antiretroviral Therapy among HIV-1 Infected Adults in the Niger Delta Region of Nigeria
- PMID: 25933356
- PMCID: PMC4416715
- DOI: 10.1371/journal.pone.0125665
Factors Associated with Timing of Initiation of Antiretroviral Therapy among HIV-1 Infected Adults in the Niger Delta Region of Nigeria
Abstract
Introduction: Based on growing evidence mainly from countries outside Sub-Saharan Africa, the World Health Organisation (WHO) now recommends initiation of antiretroviral therapy (ART) in HIV-infected individuals in developing countries when CD4 cell count (CD4+) is ≤ 500 cells/ul. Nigeria accounts for about 14% of the estimated HIV/AIDS burden in Sub-Saharan Africa. We evaluated the factors associated with timing of initiation of ART among treatment-ineligible HIV-infected adults from Nigeria.
Methods: We retrospectively reviewed the hospital records of ART ineligible HIV-infected adults who enrolled into HIV care between January 2008 and December 2012 at two major tertiary hospitals in Bayelsa State, South-South Nigeria. Demographic, clinical and laboratories data were obtained at presentation, at each subsequent visit at 6 monthly intervals and at time of initiation of ART. Cox proportional regression and Kaplan-Meier survival analysis were used to evaluate independent predictors of time to initiation of ART.
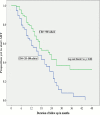
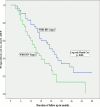
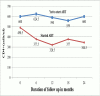
Results: Amongst the 280 study participants, 70.6% were females, 62.6% had CD4+ ≥500 cells/ul, 48.4% had WHO HIV Stage 1 disease and 34.3% were lost to follow up. In a cohort of 180 participants followed up for ≥3 months, participants with CD4+ of 351-500 cells/ul and stage 2 disease were more likely to start ART earlier than those with CD4+ > 500 cells/ul (Hazard ratio [HR]-1.7, 95% confidence interval [CI] of 1.0-2.9) and stage 1 disease (HR-2.3 (95% CI-1.3-4.2) respectively. HIV-infected adults with faster CD4+ decay required earlier ART initiation, especially in the first year of follow up.
Conclusion: ART-ineligible HIV-infected adults on follow up in South-South Nigeria are more likely to require earlier initiation of ART if they have stage 2 HIV disease or CD4+ ≤500 cells/ul at presentation. Our findings suggest faster progression of HIV-disease in these groups of individuals and corroborate the growing evidence in support for earlier initiation of ART.
Conflict of interest statement
Figures
Similar articles
-
From CD4-Based Initiation to Treating All HIV-Infected Adults Immediately: An Evidence-Based Meta-analysis.Front Immunol. 2018 Feb 13;9:212. doi: 10.3389/fimmu.2018.00212. eCollection 2018. Front Immunol. 2018. PMID: 29487595 Free PMC article.
-
Outcomes of Nigeria's HIV/AIDS Treatment Program for Patients Initiated on Antiretroviral Treatment between 2004-2012.PLoS One. 2016 Nov 9;11(11):e0165528. doi: 10.1371/journal.pone.0165528. eCollection 2016. PLoS One. 2016. PMID: 27829033 Free PMC article.
-
High rates of loss to follow-up during the first year of pre-antiretroviral therapy for HIV patients at sites providing pre-ART care in Nigeria, 2004-2012.PLoS One. 2017 Sep 1;12(9):e0183823. doi: 10.1371/journal.pone.0183823. eCollection 2017. PLoS One. 2017. PMID: 28863160 Free PMC article.
-
Decreasing time to antiretroviral therapy initiation after HIV diagnosis in a clinic-based observational cohort study in four African countries.J Int AIDS Soc. 2020 Feb;23(2):e25446. doi: 10.1002/jia2.25446. J Int AIDS Soc. 2020. PMID: 32064776 Free PMC article.
-
Predictors of treatment interruption among patients on antiretroviral therapy in Akwa Ibom, Nigeria: outcomes after 12 months.AIDS Care. 2023 Jan;35(1):114-122. doi: 10.1080/09540121.2022.2093826. Epub 2022 Jun 28. AIDS Care. 2023. PMID: 35765160 Review.
Cited by
-
Timing and Predictors of Initiation on Antiretroviral Therapy Among Newly-Diagnosed HIV-Infected Persons in South Africa.AIDS Behav. 2019 Feb;23(2):375-385. doi: 10.1007/s10461-018-2222-2. AIDS Behav. 2019. PMID: 30008050 Free PMC article.
-
Current ART, determinants for virologic failure and implications for HIV drug resistance: an umbrella review.AIDS Res Ther. 2023 Oct 27;20(1):74. doi: 10.1186/s12981-023-00572-6. AIDS Res Ther. 2023. PMID: 37884997 Free PMC article.
-
HIV Infection in Portugal: Measuring the Time Between Linkage to Care and Antiretroviral Therapy Initiation.Healthcare (Basel). 2025 Jul 25;13(15):1812. doi: 10.3390/healthcare13151812. Healthcare (Basel). 2025. PMID: 40805844 Free PMC article.
References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendation for a public health approach 2013:1–272. www.who.int (accessed May 24, 2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

