Chronic renal ischemia in humans: can cell therapy repair the kidney in occlusive renovascular disease?
- PMID: 25933818
- PMCID: PMC4422975
- DOI: 10.1152/physiol.00065.2013
Chronic renal ischemia in humans: can cell therapy repair the kidney in occlusive renovascular disease?
Abstract
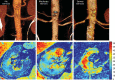
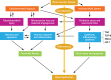
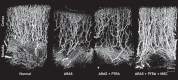
Occlusive renovascular disease caused by atherosclerotic renal artery stenosis (ARAS) elicits complex biological responses that eventually lead to loss of kidney function. Recent studies indicate a complex interplay of oxidative stress, endothelial dysfunction, and activation of fibrogenic and inflammatory cytokines as a result of atherosclerosis, hypoxia, and renal hypoperfusion in this disorder. Human studies emphasize the limits of the kidney adaptation to reduced blood flow, eventually leading to renal hypoxia with activation of inflammatory and fibrogenic pathways. Several randomized prospective clinical trials show that stent revascularization alone in patients with atherosclerotic renal artery stenosis provides little additional benefit to medical therapy once these processes have developed and solidified. Experimental data now support developing adjunctive cell-based measures to support angiogenesis and anti-inflammatory renal repair mechanisms. These data encourage the study of endothelial progenitor cells and/or mesenchymal stem/stromal cells for the repair of damaged kidney tissue.
©2015 Int. Union Physiol. Sci./Am. Physiol. Soc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
Similar articles
-
Renal artery stenosis: medical versus interventional therapy.Curr Cardiol Rep. 2013 Oct;15(10):409. doi: 10.1007/s11886-013-0409-8. Curr Cardiol Rep. 2013. PMID: 23990274 Review.
-
Mesenchymal stem cells and chronic renal artery stenosis.Am J Physiol Renal Physiol. 2016 Jan 1;310(1):F6-9. doi: 10.1152/ajprenal.00341.2015. Epub 2015 Nov 4. Am J Physiol Renal Physiol. 2016. PMID: 26538439 Review.
-
Paradigm Shifts in Atherosclerotic Renovascular Disease: Where Are We Now?J Am Soc Nephrol. 2015 Sep;26(9):2074-80. doi: 10.1681/ASN.2014121274. Epub 2015 Apr 13. J Am Soc Nephrol. 2015. PMID: 25868641 Free PMC article. Review.
-
Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease.J Am Soc Nephrol. 2017 Sep;28(9):2777-2785. doi: 10.1681/ASN.2017020151. Epub 2017 May 1. J Am Soc Nephrol. 2017. PMID: 28461553 Free PMC article. Clinical Trial.
-
The Role of Hypoxia in Ischemic Chronic Kidney Disease.Semin Nephrol. 2019 Nov;39(6):589-598. doi: 10.1016/j.semnephrol.2019.10.008. Semin Nephrol. 2019. PMID: 31836041 Review.
Cited by
-
Mesenchymal stem cells in ischemic tissue regeneration.World J Stem Cells. 2023 Feb 26;15(2):16-30. doi: 10.4252/wjsc.v15.i2.16. World J Stem Cells. 2023. PMID: 36909782 Free PMC article. Review.
-
Atherosclerotic renal artery stenosis is associated with elevated cell cycle arrest markers related to reduced renal blood flow and postcontrast hypoxia.Nephrol Dial Transplant. 2016 Nov;31(11):1855-1863. doi: 10.1093/ndt/gfw265. Epub 2016 Jul 29. Nephrol Dial Transplant. 2016. PMID: 27474749 Free PMC article.
-
Cell-based regenerative medicine for renovascular disease.Trends Mol Med. 2021 Sep;27(9):882-894. doi: 10.1016/j.molmed.2021.06.004. Epub 2021 Jun 25. Trends Mol Med. 2021. PMID: 34183258 Free PMC article. Review.
-
The application of extracorporeal shock wave therapy on stem cells therapy to treat various diseases.Stem Cell Res Ther. 2024 Aug 26;15(1):271. doi: 10.1186/s13287-024-03888-w. Stem Cell Res Ther. 2024. PMID: 39183302 Free PMC article. Review.
-
Vitamin D Deficiency Cause Gender Specific Alterations of Renal Arterial Function in a Rodent Model.Nutrients. 2021 Feb 22;13(2):704. doi: 10.3390/nu13020704. Nutrients. 2021. PMID: 33671779 Free PMC article.
References
-
- Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000. - PubMed
-
- Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1063–F1068, 1994. - PubMed
-
- Brezis M, Rosen S. Hypoxia of the renal medulla: its implications for disease. N Engl J Med 332: 647–655, 1995. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

