Vaginal drug distribution modeling
- PMID: 25933938
- PMCID: PMC4600641
- DOI: 10.1016/j.addr.2015.04.017
Vaginal drug distribution modeling
Abstract
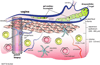
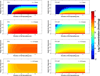
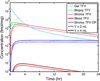
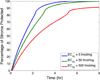
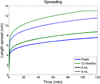
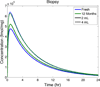
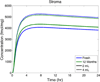
This review presents and applies fundamental mass transport theory describing the diffusion and convection driven mass transport of drugs to the vaginal environment. It considers sources of variability in the predictions of the models. It illustrates use of model predictions of microbicide drug concentration distribution (pharmacokinetics) to gain insights about drug effectiveness in preventing HIV infection (pharmacodynamics). The modeling compares vaginal drug distributions after different gel dosage regimens, and it evaluates consequences of changes in gel viscosity due to aging. It compares vaginal mucosal concentration distributions of drugs delivered by gels vs. intravaginal rings. Finally, the modeling approach is used to compare vaginal drug distributions across species with differing vaginal dimensions. Deterministic models of drug mass transport into and throughout the vaginal environment can provide critical insights about the mechanisms and determinants of such transport. This knowledge, and the methodology that obtains it, can be applied and translated to multiple applications, involving the scientific underpinnings of vaginal drug distribution and the performance evaluation and design of products, and their dosage regimens, that achieve it.
Keywords: Gel; HIV; Microbicides; Modeling; Mucosa; Ring; Vagina.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Vaginal deployment and tenofovir delivery by microbicide gels.Drug Deliv Transl Res. 2015 Jun;5(3):279-94. doi: 10.1007/s13346-015-0227-1. Drug Deliv Transl Res. 2015. PMID: 25874971 Free PMC article.
-
Recent advances on anti-HIV vaginal delivery systems development.Adv Drug Deliv Rev. 2015 Sep 15;92:123-45. doi: 10.1016/j.addr.2015.03.015. Epub 2015 Apr 7. Adv Drug Deliv Rev. 2015. PMID: 25858666 Review.
-
Acceptability and preferences for vaginal dosage forms intended for prevention of HIV or HIV and pregnancy.Adv Drug Deliv Rev. 2015 Sep 15;92:146-54. doi: 10.1016/j.addr.2015.02.004. Epub 2015 Feb 19. Adv Drug Deliv Rev. 2015. PMID: 25703190 Review.
-
Semi-solid gels function as physical barriers to human immunodeficiency virus transport in vitro.Antiviral Res. 2010 Nov;88(2):143-51. doi: 10.1016/j.antiviral.2010.08.006. Epub 2010 Aug 13. Antiviral Res. 2010. PMID: 20709109 Free PMC article.
-
Development and verification of mechanistic vaginal absorption and metabolism model to predict systemic exposure after vaginal ring and gel application.Br J Clin Pharmacol. 2024 Jun;90(6):1428-1449. doi: 10.1111/bcp.16029. Epub 2024 Mar 7. Br J Clin Pharmacol. 2024. PMID: 38450818
Cited by
-
Optimization of a Vaginal Suppository Formulation to Deliver SHetA2 as a Novel Treatment for Cervical Dysplasia.J Pharm Sci. 2018 Feb;107(2):638-646. doi: 10.1016/j.xphs.2017.09.018. Epub 2017 Oct 6. J Pharm Sci. 2018. PMID: 28989018 Free PMC article.
-
Deducing Mucosal Pharmacokinetics and Pharmacodynamics of the Anti-HIV Molecule Tenofovir from Measurements in Blood.Sci Rep. 2019 Jan 14;9(1):82. doi: 10.1038/s41598-018-36004-z. Sci Rep. 2019. PMID: 30643165 Free PMC article.
-
FbD directed fabrication and investigation of luliconazole based SLN gel for the amelioration of candidal vulvovaginitis: a 2 T (thermosensitive & transvaginal) approach.Saudi J Biol Sci. 2021 Jan;28(1):317-326. doi: 10.1016/j.sjbs.2020.10.005. Epub 2020 Oct 16. Saudi J Biol Sci. 2021. PMID: 33424312 Free PMC article.
-
Drug and therapeutic intravaginal delivery targeting diseases in the female reproductive tract: A mathematical modeling perspective.J Control Release. 2025 Aug 10;384:113924. doi: 10.1016/j.jconrel.2025.113924. Epub 2025 Jun 2. J Control Release. 2025. PMID: 40466740 Review.
-
Design and Characterization of a Novel Series of Geometrically Complex Intravaginal Rings with Digital Light Synthesis.Adv Mater Technol. 2020 Aug;5(8):2000261. doi: 10.1002/admt.202000261. Epub 2020 Jun 23. Adv Mater Technol. 2020. PMID: 33072856 Free PMC article.
References
-
- Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Human Reproduction. 2006;21:1618–1622. - PubMed
-
- Masters WH, Johnson VE. Human Sexual Response. Boston: Little, Brown; 1966.
-
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. - PubMed
-
- Harrison PF, Rosenberg Z, Bowcut J. Topical microbicides for disease prevention: status and challenges. Clin Infect Dis. 2003;36:1290–1294. - PubMed
-
- Faundes A, Brache V, Alvarez F. Pros and cons of vaginal rings for contraceptive hormone delivery. Am J Drug Deliv. 2004;2:241–250.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

