Combined Crystal Structure of a Type I Cohesin: MUTATION AND AFFINITY BINDING STUDIES REVEAL STRUCTURAL DETERMINANTS OF COHESIN-DOCKERIN SPECIFICITIES
- PMID: 25934389
- PMCID: PMC4481221
- DOI: 10.1074/jbc.M115.653303
Combined Crystal Structure of a Type I Cohesin: MUTATION AND AFFINITY BINDING STUDIES REVEAL STRUCTURAL DETERMINANTS OF COHESIN-DOCKERIN SPECIFICITIES
Abstract
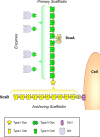
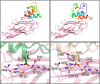
Cohesin-dockerin interactions orchestrate the assembly of one of nature's most elaborate multienzyme complexes, the cellulosome. Cellulosomes are produced exclusively by anaerobic microbes and mediate highly efficient hydrolysis of plant structural polysaccharides, such as cellulose and hemicellulose. In the canonical model of cellulosome assembly, type I dockerin modules of the enzymes bind to reiterated type I cohesin modules of a primary scaffoldin. Each type I dockerin contains two highly conserved cohesin-binding sites, which confer quaternary flexibility to the multienzyme complex. The scaffoldin also bears a type II dockerin that anchors the entire complex to the cell surface by binding type II cohesins of anchoring scaffoldins. In Bacteroides cellulosolvens, however, the organization of the cohesin-dockerin types is reversed, whereby type II cohesin-dockerin pairs integrate the enzymes into the primary scaffoldin, and type I modules mediate cellulosome attachment to an anchoring scaffoldin. Here, we report the crystal structure of a type I cohesin from B. cellulosolvens anchoring scaffoldin ScaB to 1.84-Å resolution. The structure resembles other type I cohesins, and the putative dockerin-binding site, centered at β-strands 3, 5, and 6, is likely to be conserved in other B. cellulosolvens type I cohesins. Combined computational modeling, mutagenesis, and affinity-based binding studies revealed similar hydrogen-bonding networks between putative Ser/Asp recognition residues in the dockerin at positions 11/12 and 45/46, suggesting that a dual-binding mode is not exclusive to the integration of enzymes into primary cellulosomes but can also characterize polycellulosome assembly and cell-surface attachment. This general approach may provide valuable structural information of the cohesin-dockerin interface, in lieu of a definitive crystal structure.
Keywords: cellulase; cellulose; cellulosome; computational biology; computer modeling; protein-protein interaction.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
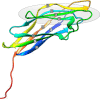


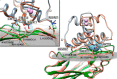
References
-
- Bayer E. A., Belaich J.-P., Shoham Y., Lamed R. (2004) The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58, 521–554 - PubMed
-
- Fontes C. M., Gilbert H. J. (2010) Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 79, 655–681 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

