A Conserved Residue Cluster That Governs Kinetics of ATP-dependent Gating of Kir6.2 Potassium Channels
- PMID: 25934393
- PMCID: PMC4505460
- DOI: 10.1074/jbc.M114.631960
A Conserved Residue Cluster That Governs Kinetics of ATP-dependent Gating of Kir6.2 Potassium Channels
Abstract
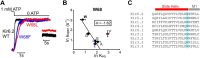
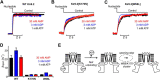
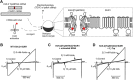
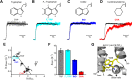
ATP-sensitive potassium (KATP) channels are heteromultimeric complexes of an inwardly rectifying Kir channel (Kir6.x) and sulfonylurea receptors. Their regulation by intracellular ATP and ADP generates electrical signals in response to changes in cellular metabolism. We investigated channel elements that control the kinetics of ATP-dependent regulation of KATP (Kir6.2 + SUR1) channels using rapid concentration jumps. WT Kir6.2 channels re-open after rapid washout of ATP with a time constant of ∼60 ms. Extending similar kinetic measurements to numerous mutants revealed fairly modest effects on gating kinetics despite significant changes in ATP sensitivity and open probability. However, we identified a pair of highly conserved neighboring amino acids (Trp-68 and Lys-170) that control the rate of channel opening and inhibition in response to ATP. Paradoxically, mutations of Trp-68 or Lys-170 markedly slow the kinetics of channel opening (500 and 700 ms for W68L and K170N, respectively), while increasing channel open probability. Examining the functional effects of these residues using φ value analysis revealed a steep negative slope. This finding implies that these residues play a role in lowering the transition state energy barrier between open and closed channel states. Using unnatural amino acid incorporation, we demonstrate the requirement for a planar amino acid at Kir6.2 position 68 for normal channel gating, which is potentially necessary to localize the ϵ-amine of Lys-170 in the phosphatidylinositol 4,5-bisphosphate-binding site. Overall, our findings identify a discrete pair of highly conserved residues with an essential role for controlling gating kinetics of Kir channels.
Keywords: KATP channel; PIP2; diabetes; gating; ion channel; kinetics; ligand-dependent gating; potassium channel.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 - PubMed
-
- Phillips L. R., Enkvetchakul D., Nichols C. G. (2003) Gating dependence of inner pore access in inward rectifier K+ channels. Neuron 37, 953–962 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

