Non-neuronal and neuronal BACE1 elevation in association with angiopathic and leptomeningeal β-amyloid deposition in the human brain
- PMID: 25934480
- PMCID: PMC4428107
- DOI: 10.1186/s12883-015-0327-z
Non-neuronal and neuronal BACE1 elevation in association with angiopathic and leptomeningeal β-amyloid deposition in the human brain
Abstract
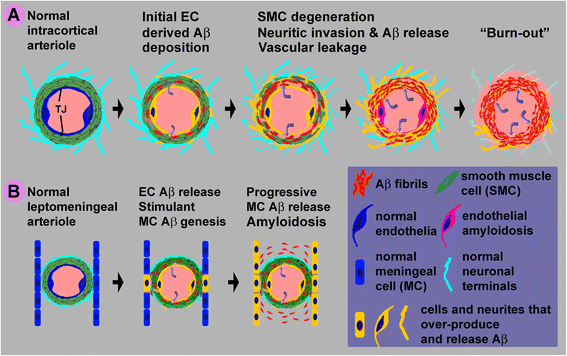
Background: Cerebral amyloid angiopathy (CAA) refers to the deposition of β-amyloid (Aβ) peptides in the wall of brain vasculature, commonly involving capillaries and arterioles. Also being considered a part of CAA is the Aβ deposition in leptomeninge. The cellular origin of angiopathic Aβ and the pathogenic course of CAA remain incompletely understood.
Methods: The present study was aimed to explore the pathogenic course of CAA in the human cerebrum via examination of changes in β-secretase-1 (BACE1), the obligatory Aβ producing enzyme, relative to Aβ and other cellular markers, by neuroanatomical and biochemical characterizations with postmortem brain samples and primary cell cultures.
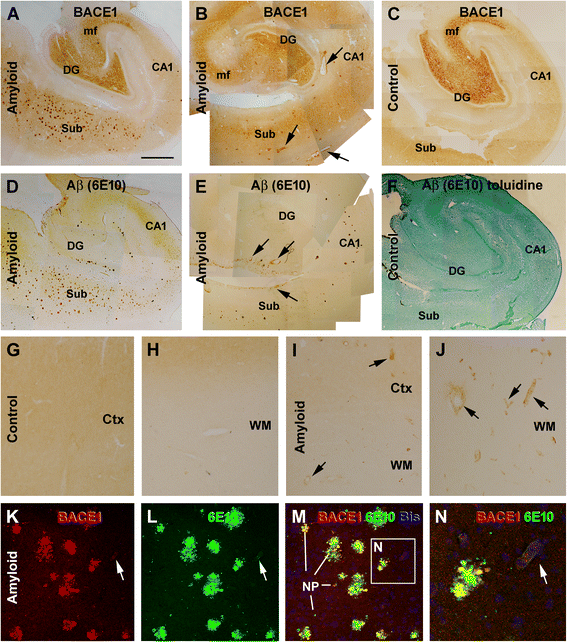
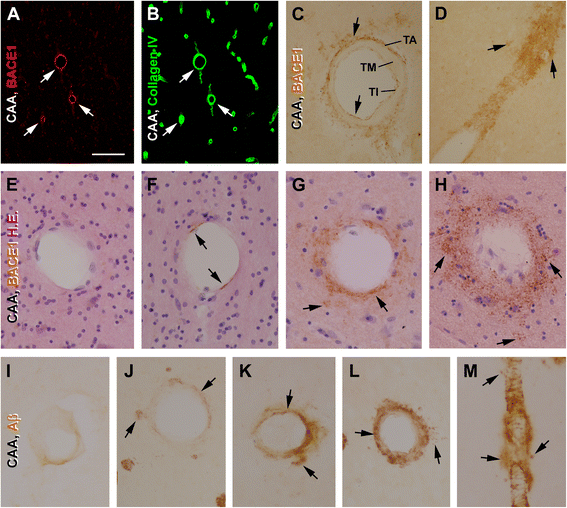
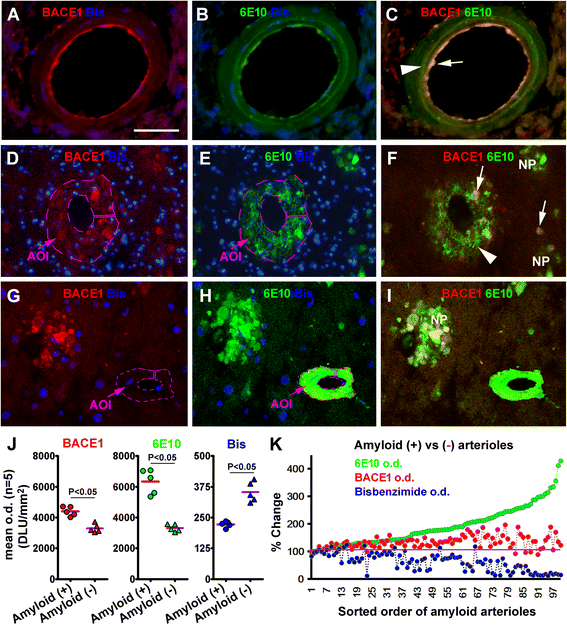
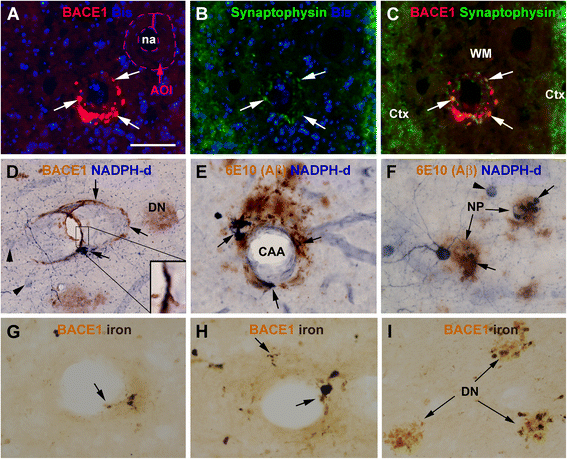
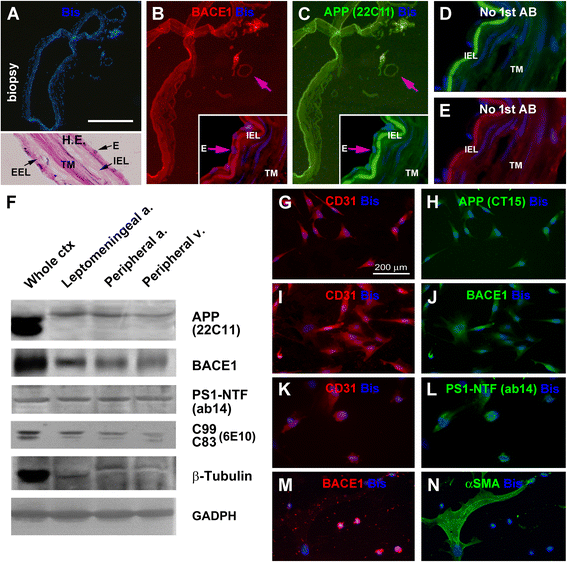
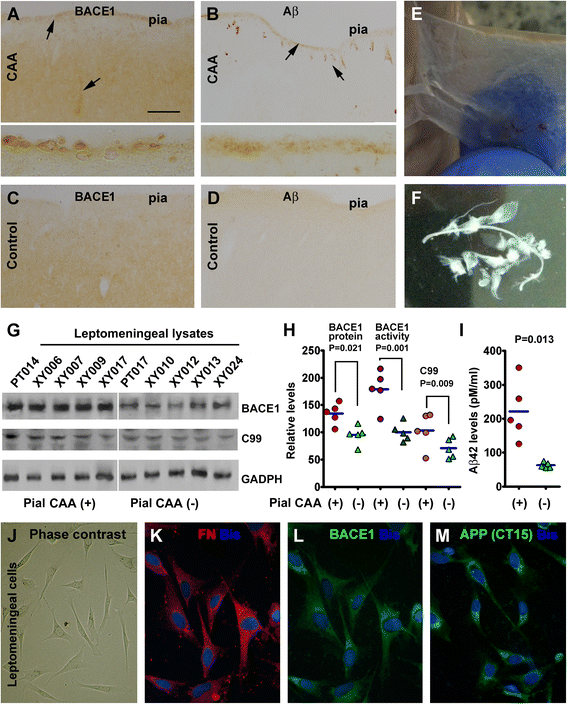
Results: Immunoreactivity (IR) for BACE1 was essentially not visible at vasculature in cases without cerebral amyloidosis (control group, n = 15, age = 86.1 ± 10.3 year). In cases with brain amyloid pathology (n = 15, age = 78.7 ± 12.7 year), increased BACE1 IR was identified locally at capillaries, arterioles and along the pia, localizing to endothelia, perivascular dystrophic neurites and meningeal cells, and often coexisting with vascular iron deposition. Double immunofluorescence with densitometric analysis confirmed a site-specific BACE1 elevation at cerebral arterioles in the development of vascular Aβ deposition. Levels of BACE1 protein, activity and its immediate product (C99) were elevated in leptomeningeal lysates from cases with CAA relative to controls. The expression of BACE1 and other amyloidogenic proteins in the endothelial and meningeal cells was confirmed in primary cultures prepared from human leptomeningeal and arteriolar biopsies.
Conclusion: These results suggest that BACE1 elevation in the endothelia and perivascular neurites may be involved in angiopathic Aβ deposition, while BACE1 elevation in meningeal cells might contribute Aβ to leptomeningeal amyloidosis.
Figures
References
-
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

