Effects of cytarabine on activation of human T cells - cytarabine has concentration-dependent effects that are modulated both by valproic acid and all-trans retinoic acid
- PMID: 25934555
- PMCID: PMC4422044
- DOI: 10.1186/s40360-015-0012-2
Effects of cytarabine on activation of human T cells - cytarabine has concentration-dependent effects that are modulated both by valproic acid and all-trans retinoic acid
Abstract
Background: Cytarabine is used in the treatment of acute myeloid leukemia (AML). Low-dose cytarabine can be combined with valproic acid and all-trans retinoic acid (ATRA) as AML-stabilizing treatment. We have investigated the possible risk of immunotoxicity by this combination. We examined the effects of cytarabine combined with valproic acid and ATRA on in vitro activated human T cells, and we tested cytarabine at concentrations reached during in vivo treatment with high doses, conventional doses and low doses.
Methods: T cells derived from blood donors were activated in vitro in cell culture medium alone or supplemented with ATRA (1 μM), valproic acid (500 or 1000 μM) or cytarabine (0.01-44 μM). Cell characteristics were assessed by flow cytometry. Supernatants were analyzed for cytokines by ELISA or Luminex. Effects on primary human AML cell viability and proliferation of low-dose cytarabine (0.01-0.5 μM) were also assessed. Statistical tests include ANOVA and Cluster analyses.
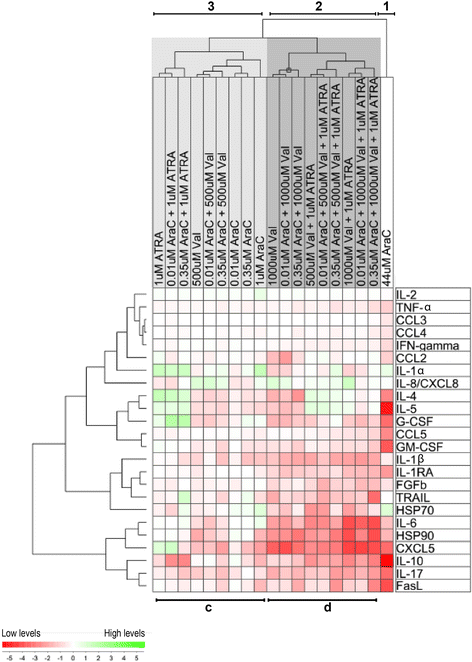
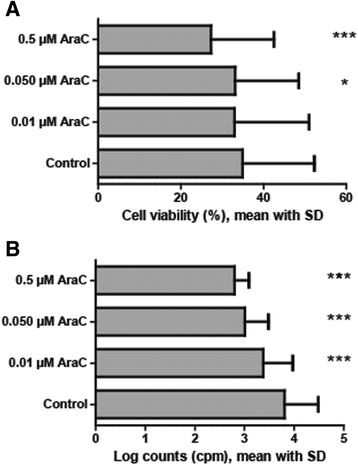
Results: Only cytarabine 44 μM had both antiproliferative and proapoptotic effects. Additionally, this concentration increased the CD4:CD8 T cell ratio, prolonged the expression of the CD69 activation marker, inhibited CD95L and heat shock protein (HSP) 90 release, and decreased the release of several cytokines. In contrast, the lowest concentrations (0.35 and 0.01 μM) did not have or showed minor antiproliferative or cytotoxic effects, did not alter activation marker expression (CD38, CD69) or the release of CD95L and HSP90, but inhibited the release of certain T cell cytokines. Even when these lower cytarabine concentrations were combined with ATRA and/or valproic acid there was still no or minor effects on T cell viability. However, these combinations had strong antiproliferative effects, the expression of both CD38 and CD69 was altered and there was a stronger inhibition of the release of FasL, HSP90 as well as several cytokines. Cytarabine (0.01-0.05 μM) showed a dose-dependent antiproliferative effect on AML cells, and in contrast to the T cells this effect reached statistical significance even at 0.01 μM.
Conclusions: Even low levels of cytarabine, and especially when combined with ATRA and valproic acid, can decrease T cell viability, alter activation-induced membrane-molecule expression and decrease the cytokine release.
Figures






References
-
- Liseth K, Sjo M, Paulsen K, Bruserud O, Ersvaer E. Early pre-engraftment, functional, in vitro responsiveness of T lymphocytes in allotransplanted, acute leukemia patients: proliferation and release of a broad profile of cytokines, possibly predictive of graft-versus-host disease. Eur Cytokine Netw. 2010;21(1):40–49. - PubMed
-
- Porrata LF, Litzow MR, Tefferi A, Letendre L, Kumar S, Geyer SM, et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16(7):1311–1318. doi: 10.1038/sj.leu.2402503. - DOI - PubMed
-
- Parkman R, Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12(9):919–927. doi: 10.1016/j.bbmt.2006.05.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

