Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons
- PMID: 25934637
- PMCID: PMC4428232
- DOI: 10.1186/s12990-015-0023-4
Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons
Abstract
Background: Hydrogen sulfide (H2S) is oxidized to polysulfide. Recent reports show that this sulfur compound modulates various biological functions. We have reported that H2S is involved in inflammatory pain in mice. On the other hand, little is known about the functional role of polysulfide in sensory neurons. Here we show that polysulfide selectively stimulates nociceptive TRPA1 and evokes acute pain, using TRPA1-gene deficient mice (TRPA1(-/-)), a heterologous expression system and a TRPA1-expressing cell line.
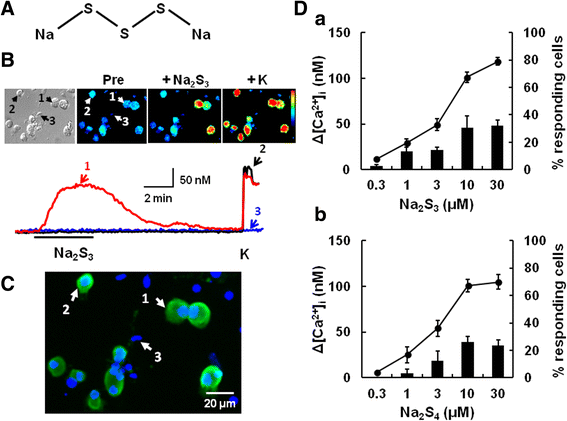
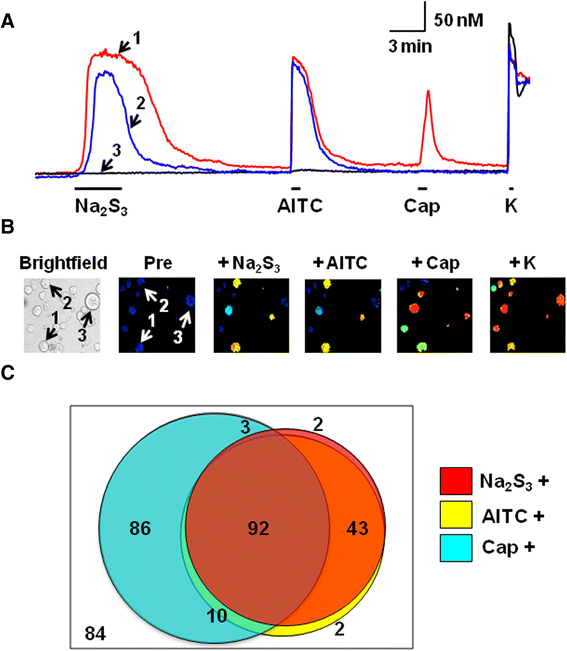
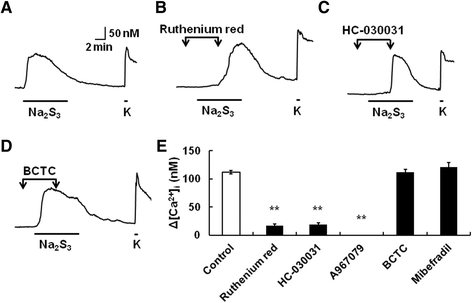
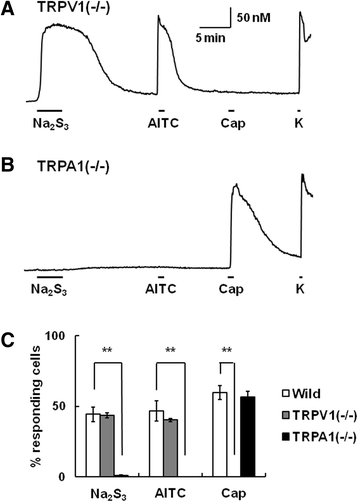
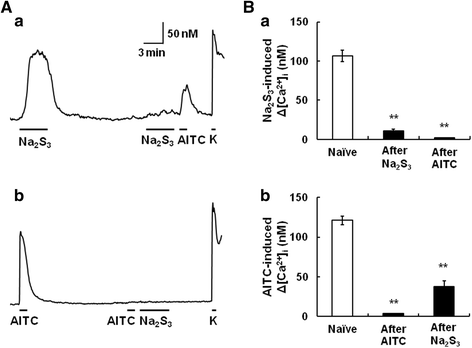
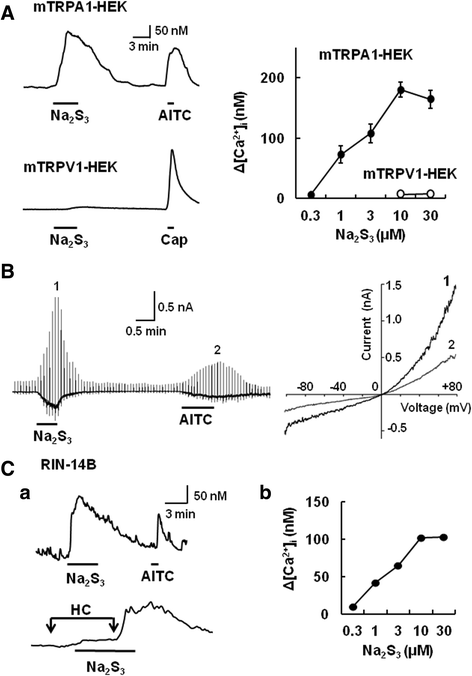
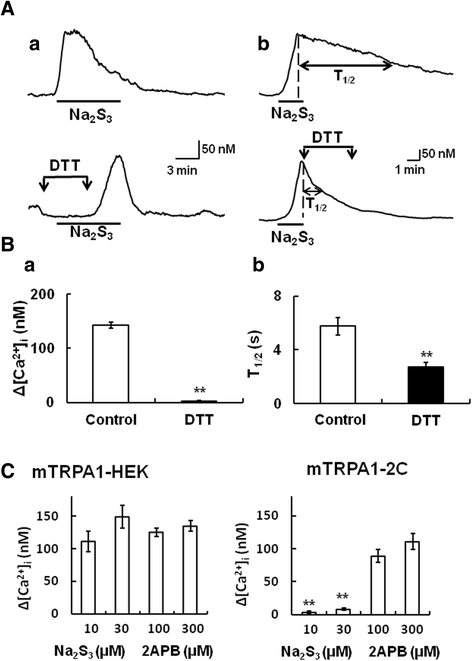
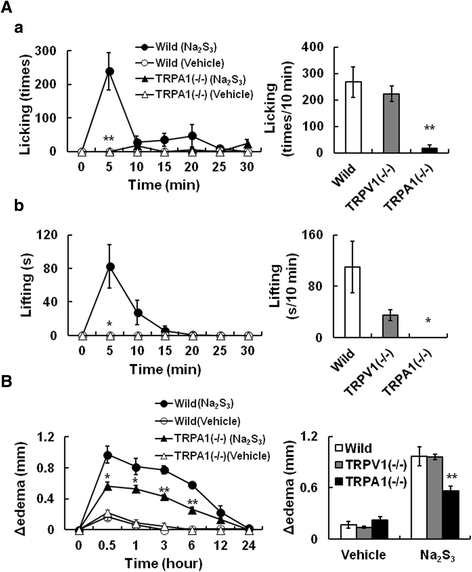
Results: In wild-type mouse sensory neurons, polysulfide elevated the intracellular Ca concentration ([Ca(2+)]i) in a dose-dependent manner. The half maximal effective concentration (EC50) of polysulfide was less than one-tenth that of H2S. The [Ca(2+)]i responses to polysulfide were observed in neurons responsive to TRPA1 agonist and were inhibited by blockers of TRPA1 but not of TRPV1. Polysulfide failed to evoke [Ca(2+)]i increases in neurons from TRPA1(-/-) mice. In RIN-14B cells, constitutively expressing rat TRPA1, polysulfide evoked [Ca(2+)]i increases with the same EC50 value as in sensory neurons. Heterologously expressed mouse TRPA1 was activated by polysulfide and that was suppressed by dithiothreitol. Analyses of the TRPA1 mutant channel revealed that cysteine residues located in the internal domain were related to the sensitivity to polysulfide. Intraplantar injection of polysulfide into the mouse hind paw induced acute pain and edema which were significantly less than in TRPA1(-/-) mice.
Conclusions: The present data suggest that polysulfide functions as pronociceptive substance through the activation of TRPA1 in sensory neurons. Since the potency of polysulfide is higher than parental H2S and this sulfur compound is generated under pathophysiological conditions, it is suggested that polysulfide acts as endogenous ligand for TRPA1. Therefore, TRPA1 may be a promising therapeutic target for endogenous sulfur compound-related algesic action.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

