Dietary ω-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease
- PMID: 25934765
- PMCID: PMC4486221
- DOI: 10.3324/haematol.2015.124586
Dietary ω-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease
Abstract
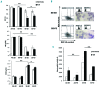
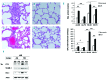
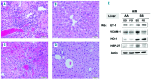
The anemia of sickle cell disease is associated with a severe inflammatory vasculopathy and endothelial dysfunction, which leads to painful and life-threatening clinical complications. Growing evidence supports the anti-inflammatory properties of ω-3 fatty acids in clinical models of endothelial dysfunction. Promising but limited studies show potential therapeutic effects of ω-3 fatty acid supplementation in sickle cell disease. Here, we treated humanized healthy and sickle cell mice for 6 weeks with ω-3 fatty acid diet (fish-oil diet). We found that a ω-3 fatty acid diet: (i) normalizes red cell membrane ω-6/ω-3 ratio; (ii) reduces neutrophil count; (iii) decreases endothelial activation by targeting endothelin-1 and (iv) improves left ventricular outflow tract dimensions. In a hypoxia-reoxygenation model of acute vaso-occlusive crisis, a ω-3 fatty acid diet reduced systemic and local inflammation and protected against sickle cell-related end-organ injury. Using isolated aortas from sickle cell mice exposed to hypoxia-reoxygenation, we demonstrated a direct impact of a ω-3 fatty acid diet on vascular activation, inflammation, and anti-oxidant systems. Our data provide the rationale for ω-3 dietary supplementation as a therapeutic intervention to reduce vascular dysfunction in sickle cell disease.
Copyright© Ferrata Storti Foundation.
Figures



References
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. - PubMed
-
- De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Semin Thromb Hemost. 2011;37(3):226–236. - PubMed
-
- Massaro M, Scoditti E, Carluccio MA, De Caterina R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):109–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

