Topical treatment with fresh human milk versus emollient on atopic eczema spots in young children: a small, randomized, split body, controlled, blinded pilot study
- PMID: 25935520
- PMCID: PMC4424556
- DOI: 10.1186/s12895-015-0027-9
Topical treatment with fresh human milk versus emollient on atopic eczema spots in young children: a small, randomized, split body, controlled, blinded pilot study
Abstract
Background: Public health nurses report on effects of fresh human milk as treatment for conjunctivitis, rhinitis and atopic eczema (AE), the latter being highly prevalent in early childhood. Emollients and topical corticosteroids are first line treatment of AE. As many caregivers have steroid phobia, alternative treatment options for mild AE are of interest. The aim of this small pilot study was to assess the potential effects and risks of applying fresh human milk locally on eczema spots in children with AE.
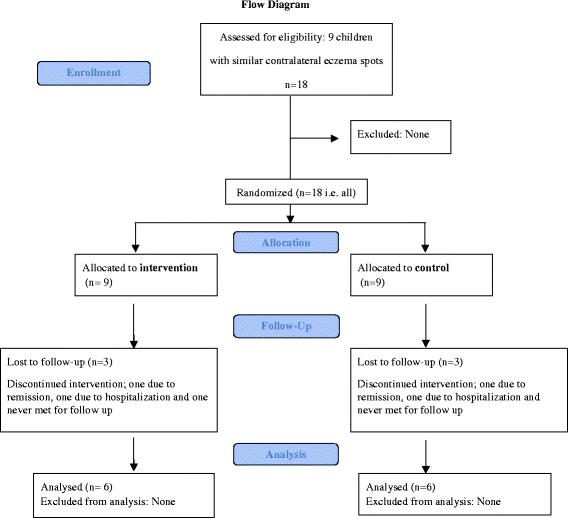
Methods: This was a split body, controlled, randomized and physician blinded pilot study, of children with AE with two similar contralateral eczema spots having a mother breastfeeding the child or a sibling. Fresh expressed milk and emollient was applied on the intervention spot and emollient alone on the control area, three times a day for four weeks. The severity and area of the eczema spots was evaluated weekly, and samples from milk and the spots were analysed weekly with respect to bacterial colonisation.
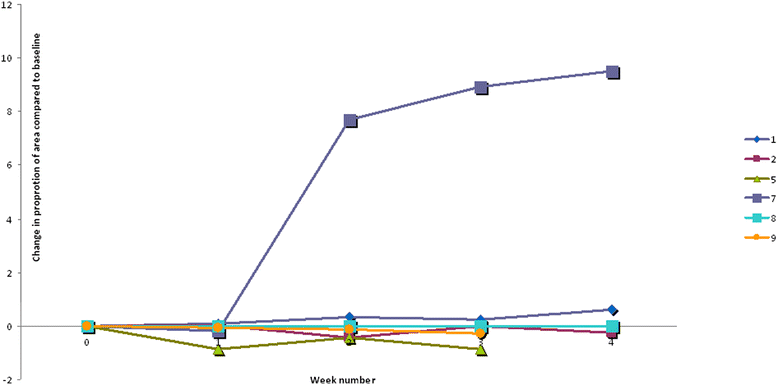
Results: Of nine patients included, six completed the study. Mean age at inclusion was 18.5 months. The spots examined were localized on the arms, legs or cheeks. The spots were similar in severity, but differed in area. In one patient the eczema ceased after inclusion. In four patients both control and intervention areas increased during the intervention. The relative change in eczema area compared to baseline showed less increase in the intervention spots in two patients, whereas the opposite was observed in three. In four children Staphylococcus aureus was found in their eczema once or more. In three of the 28 human milk samples, Staphylococcus aureus, alfa haemolytic streptococci or coagulase negative staphylococci were detected. Staphylococcus aureus was found once both in human milk and in the eczema spots, no clinical signs of infection were however observed. No secondary infection due to milk application was detected.
Conclusion: In this small pilot study, no effect was found on eczema spots treated with topical application of fresh human milk. (ClinicalTrials.gov Identifier, NCT02381028 ).
Figures
References
-
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32. doi: 10.1016/j.jaad.2014.03.023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

