Spatiotemporal control of opioid signaling and behavior
- PMID: 25937173
- PMCID: PMC4441608
- DOI: 10.1016/j.neuron.2015.03.066
Spatiotemporal control of opioid signaling and behavior
Abstract
Optogenetics is now a widely accepted tool for spatiotemporal manipulation of neuronal activity. However, a majority of optogenetic approaches use binary on/off control schemes. Here, we extend the optogenetic toolset by developing a neuromodulatory approach using a rationale-based design to generate a Gi-coupled, optically sensitive, mu-opioid-like receptor, which we term opto-MOR. We demonstrate that opto-MOR engages canonical mu-opioid signaling through inhibition of adenylyl cyclase, activation of MAPK and G protein-gated inward rectifying potassium (GIRK) channels and internalizes with kinetics similar to that of the mu-opioid receptor. To assess in vivo utility, we expressed a Cre-dependent viral opto-MOR in RMTg/VTA GABAergic neurons, which led to a real-time place preference. In contrast, expression of opto-MOR in GABAergic neurons of the ventral pallidum hedonic cold spot led to real-time place aversion. This tool has generalizable application for spatiotemporal control of opioid signaling and, furthermore, can be used broadly for mimicking endogenous neuronal inhibition pathways.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
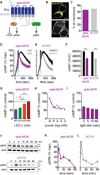
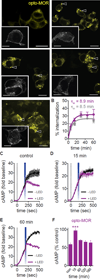
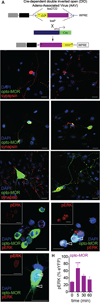



Comment in
-
Using opioid receptors to expand the chemogenetic and optogenetic toolbox.Neuron. 2015 May 20;86(4):853-855. doi: 10.1016/j.neuron.2015.05.014. Neuron. 2015. PMID: 25996128
References
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. - PubMed
-
- Barish PA, Xu Y, Li J, Sun J, Jarajapu YPR, Ogle WO. Design and functional evaluation of an optically active µ-opioid receptor. Eur. J. Pharmacol. 2013;705:42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

