Subchronic phencyclidine treatment in adult mice increases GABAergic transmission and LTP threshold in the hippocampus
- PMID: 25937215
- PMCID: PMC4584174
- DOI: 10.1016/j.neuropharm.2015.04.012
Subchronic phencyclidine treatment in adult mice increases GABAergic transmission and LTP threshold in the hippocampus
Abstract
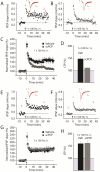
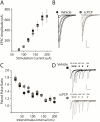
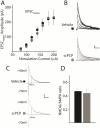
Repeated administration of non-competitive N-methyl-d-aspartate (NMDA) receptor antagonists such as phencyclidine (PCP) to rodents causes long-lasting deficits in cognition and memory, and has effects on behaviors that have been suggested to be models of the cognitive impairment associated with schizophrenia (CIAS). Despite this being a widely studied animal model, little is known about the long lasting changes in synapses and circuits that underlie the altered behaviors. Here we examined synaptic transmission ex-vivo in the hippocampus of mice after a subchronic PCP (scPCP) administration regime. We found that after at least one week of drug free washout period when mice have impaired cognitive function, the threshold for long-term potentiation (LTP) of CA1 excitatory synapses was elevated. This elevated LTP threshold was directly related to increased inhibitory input to CA1 pyramidal cells through increased activity of GABAergic neurons. These results suggest repeated PCP administration causes a long-lasting metaplastic change in the inhibitory circuits in the hippocampus that results in impaired LTP, and could contribute to the deficits in hippocampal-dependent memory in PCP-treated mice. Changes in GABA signaling have been described in patients with schizophrenia, therefore our results support using scPCP as a model of CIAS. This article is part of the Special Issue entitled 'Synaptopathy--from Biology to Therapy'.
Keywords: GABA; Hippocampus; Inhibitory postsynaptic current; Long-term potentiation; Phencyclidine.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. - PubMed
-
- Andersen JD, Pouzet B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology. 2004;29:1080–1090. - PubMed
-
- Bird ED, Spokes EG, Barnes J, Mackay AV, Iversen LL, Shepherd M. Glutamic-acid decarboxylase in schizophrenia. Lancet. 1978;1:156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

