Somatosensory substrates of flight control in bats
- PMID: 25937277
- PMCID: PMC4643944
- DOI: 10.1016/j.celrep.2015.04.001
Somatosensory substrates of flight control in bats
Abstract
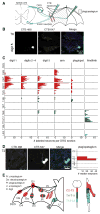
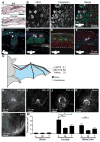
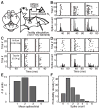
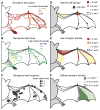
Flight maneuvers require rapid sensory integration to generate adaptive motor output. Bats achieve remarkable agility with modified forelimbs that serve as airfoils while retaining capacity for object manipulation. Wing sensory inputs provide behaviorally relevant information to guide flight; however, components of wing sensory-motor circuits have not been analyzed. Here, we elucidate the organization of wing innervation in an insectivore, the big brown bat, Eptesicus fuscus. We demonstrate that wing sensory innervation differs from other vertebrate forelimbs, revealing a peripheral basis for the atypical topographic organization reported for bat somatosensory nuclei. Furthermore, the wing is innervated by an unusual complement of sensory neurons poised to report airflow and touch. Finally, we report that cortical neurons encode tactile and airflow inputs with sparse activity patterns. Together, our findings identify neural substrates of somatosensation in the bat wing and imply that evolutionary pressures giving rise to mammalian flight led to unusual sensorimotor projections.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ackert JE. Innervation of the integument of chiroptera. Journal of Morphology. 1914;25:301–343.
-
- Angelica-Almeida M, Casal D, Mafra M, Mascarenhas-Lemos L, Martins-Ferreira J, Ferraz-Oliveira M, Amarante J, Goyri-O’Neill J. Brachial plexus morphology and vascular supply in the wistar rat. Acta medica portuguesa. 2013;26:243–250. - PubMed
-
- Augurelle AS, Smith AM, Lejeune T, Thonnard JL. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J Neurophysiol. 2003;89:665–671. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

