Glucose-based regulation of miR-451/AMPK signaling depends on the OCT1 transcription factor
- PMID: 25937278
- PMCID: PMC4431904
- DOI: 10.1016/j.celrep.2015.04.016
Glucose-based regulation of miR-451/AMPK signaling depends on the OCT1 transcription factor
Abstract
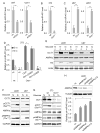
In aggressive, rapidly growing solid tumors such as glioblastoma multiforme (GBM), cancer cells face frequent dynamic changes in their microenvironment, including the availability of glucose and other nutrients. These challenges require that tumor cells have the ability to adapt in order to survive periods of nutrient/energy starvation. We have identified a reciprocal negative feedback loop mechanism in which the levels of microRNA-451 (miR-451) are negatively regulated through the phosphorylation and inactivation of its direct transcriptional activator OCT1 by 5' AMP-activated protein kinase (AMPK), which is activated by glucose depletion-induced metabolic stress. Conversely, in a glucose-rich environment, unrestrained expression of miR-451 suppresses AMPK pathway activity. These findings uncover miR-451 as a major effector of glucose-regulated AMPK signaling, allowing tumor cell adaptation to variations in nutrient availability in the tumor microenvironment.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

