FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer
- PMID: 25937522
- PMCID: PMC7508202
- DOI: 10.1016/j.ejca.2015.04.007
FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer
Abstract
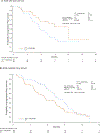
Background: The OPUS study demonstrated that addition of cetuximab to 5-fluorouracil, folinic acid and oxaliplatin (FOLFOX4) significantly improved objective response and progression-free survival (PFS) in the first-line treatment of patients with KRAS exon 2 wild-type metastatic colorectal cancer (mCRC). In patients with KRAS exon 2 mutations, a detrimental effect was seen upon addition of cetuximab to FOLFOX4. The current study reports outcomes in subgroups defined by extended RAS testing.
Patients and methods: Samples from OPUS study KRAS exon 2 wild-type tumours were reanalysed for other RAS mutations in four additional KRAS codons (exons 3-4) and six NRAS codons (exons 2-4) using BEAMing. A cutoff of ⩾5% mutant/wild-type sequences was selected to define RAS status; we also report an analysis using a cutoff based on the technical lower limit for mutation identification (0.1%).
Results: Other RAS mutations were detected in 31/118 (26%) evaluable patients. In the extended analysis of RAS wild-type tumours (n=87), objective response was significantly improved by addition of cetuximab to FOLFOX4 (58% versus 29%; odds ratio 3.33 [95% confidence interval 1.36-8.17]; P=0.0084); although limited by population size, there also appeared to be trends favouring the cetuximab arm in terms of PFS and overall survival in the RAS wild-type group compared with the RAS evaluable group. There was no evidence that patients with other RAS mutations benefited from cetuximab, but small numbers precluded precise estimations of treatment effects. In the combined population of patients with any RAS mutation (KRAS exon 2 or other RAS), a clear detrimental effect was associated with addition of cetuximab to FOLFOX4.
Conclusion: Patients with RAS-mutant mCRC, as defined by mutations in KRAS and NRAS exons 2-4, derive no benefit and may be harmed by the addition of cetuximab to FOLFOX4. Restricting cetuximab administration to patients with RAS wild-type tumours will further tailor therapy to maximise benefit.
Keywords: Cetuximab; FOLFOX4; KRAS; NRAS; OPUS; RAS.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
CB has a consultancy/advisory relationship with, and has received honoraria from, Merck Serono; C-HK has received honoraria and research funding from Merck KGaA; FC has a consultancy/advisory relationship with, and has received honoraria and research funding from, Merck Serono; H-JL has a consultancy/advisory relationship with, and has received honoraria, research funding and travel/accommodation expenses from, Merck Serono; VH has a consultancy/advisory relationship with, has participated in satellite symposia for, has provided expert testimony for, and has receiving honoraria, research funding and travel/accommodation expenses from, Merck KGaA; FB and KD are compensated employees of Merck KGaA; UK was a compensated employee of Merck KGaA up until submission of the manuscript; JHvK has a consultancy/advisory relationship with, and has received honoraria, research funding and travel/accommodation expenses from, Merck Serono; ST has a consultancy/advisory role with, and has received honoraria, lecture fees and research funding from, Merck Serono.
Figures


References
-
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663–71. - PubMed
-
- Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535–46. - PubMed
-
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. - PubMed
-
- Sorich MJ, Wiese MD, Rowland A, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2014. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

