Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment
- PMID: 25937524
- PMCID: PMC4450141
- DOI: 10.1016/j.ejrad.2015.03.017
Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment
Abstract
The recent advances in the clinical application of anti-cancer immunotherapeutic agents have opened a new arena for the treatment of advanced cancers. Cancer immunotherapy is associated with a variety of important radiographic features in the assessments of tumor response and immune-related adverse events, which calls for radiologists' awareness and in-depth knowledge on the topic. This article will provide the state-of-the art review and perspectives of cancer immunotherapy, including its molecular mechanisms, the strategies for immune-related response assessment on imaging and their pitfalls, and the emerging knowledge of radiologic manifestations of immune-related adverse events. The cutting edge clinical and radiologic investigations are presented to provide future directions.
Keywords: Cancer; Drug toxicity; Immunotherapy; Oncologic imaging; Tumor response assessment.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Dr. Nishino served as a consultant to Bristol-Myers Squibb. Dr. Hodi has served as a non-paid consultant to Bristol-Myers Squibb and has received clinical trial support from Bristol-Myers Squibb, advisor and clinical trial support from Merck, and advisor and clinical trial support from Genentech. Other authors have nothing to disclose.
Figures






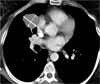
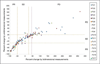


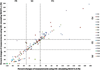





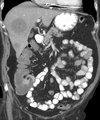


References
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. - PubMed
-
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. - PubMed
-
- Freeman GJ, Gribben JG, Boussiotis VA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

