Evaluation of dendrimer type bio-reducible polymer as a siRNA delivery carrier for cancer therapy
- PMID: 25937533
- PMCID: PMC4458390
- DOI: 10.1016/j.jconrel.2015.04.039
Evaluation of dendrimer type bio-reducible polymer as a siRNA delivery carrier for cancer therapy
Abstract
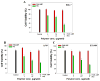
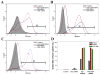
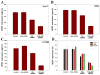
Small interfering ribonucleic acid (siRNA), 20-25 base pairs in length, can interfere with the expression of specific genes. Recently, many groups reported the therapeutic intervention of siRNA in various cancer cells. In this study, dendrimer type bio-reducible polymer (PAM-ABP) which was synthesized using arginine grafted bio-reducible poly(cystaminebisacrylamide-diaminohexane) (ABP) and polyamidoamine (PAMAM) was used to deliver anti-VEGF siRNA into cancer cell lines including human hepatocarcinoma (Huh-7), human lung adenocarcinoma (A549), and human fibrosarcoma (HT1080) cells and access their potential as a siRNA delivery carrier for cancer therapy. PAM-ABP and siRNA formed polyplexes with average diameter of 116 nm and charge of around +24.6 mV. The siRNA in the PAM-ABP/siRNA polyplex was released by 5mM DTT and heparin. VEGF gene silencing efficiency of PAM-ABP/siRNA polyplexes was shown to be more effective than PEI/siRNA polyplexes in three cell lines with the following order HT1080>A549>Huh-7.
Keywords: Bio-reducible polymer; Dendrimer; VEGF; siRNA.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

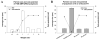
Similar articles
-
Tumor targeting RGD conjugated bio-reducible polymer for VEGF siRNA expressing plasmid delivery.Biomaterials. 2014 Aug;35(26):7543-52. doi: 10.1016/j.biomaterials.2014.05.021. Epub 2014 Jun 2. Biomaterials. 2014. PMID: 24894645 Free PMC article.
-
VEGF therapeutic gene delivery using dendrimer type bio-reducible polymer into human mesenchymal stem cells (hMSCs).J Control Release. 2015 Dec 28;220(Pt A):222-228. doi: 10.1016/j.jconrel.2015.09.018. Epub 2015 Sep 12. J Control Release. 2015. PMID: 26368313
-
Dendrimer type bio-reducible polymer for efficient gene delivery.J Control Release. 2012 Jun 28;160(3):592-600. doi: 10.1016/j.jconrel.2012.04.025. Epub 2012 Apr 23. J Control Release. 2012. PMID: 22546681
-
Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy.Int J Pharm. 2018 Jul 30;546(1-2):215-225. doi: 10.1016/j.ijpharm.2018.05.045. Epub 2018 May 19. Int J Pharm. 2018. PMID: 29787895 Review.
-
Surface Engineered Dendrimers in siRNA Delivery and Gene Silencing.Curr Pharm Des. 2017;23(20):2952-2975. doi: 10.2174/1381612823666170314104619. Curr Pharm Des. 2017. PMID: 28292248 Review.
Cited by
-
Surface-mediated delivery of siRNA from fibrin hydrogels for knockdown of the BMP-2 binding antagonist noggin.Acta Biomater. 2015 Oct;25:109-20. doi: 10.1016/j.actbio.2015.07.045. Epub 2015 Jul 30. Acta Biomater. 2015. PMID: 26234488 Free PMC article.
-
Pharmaceutical Aspects of Nanocarriers for Smart Anticancer Therapy.Pharmaceutics. 2021 Nov 5;13(11):1875. doi: 10.3390/pharmaceutics13111875. Pharmaceutics. 2021. PMID: 34834290 Free PMC article. Review.
-
Silencing of HMGA2 by siRNA Loaded Methotrexate Functionalized Polyamidoamine Dendrimer for Human Breast Cancer Cell Therapy.Genes (Basel). 2021 Jul 20;12(7):1102. doi: 10.3390/genes12071102. Genes (Basel). 2021. PMID: 34356120 Free PMC article.
-
Multifunctional Nucleus-targeting Nanoparticles with Ultra-high Gene Transfection Efficiency for In Vivo Gene Therapy.Theranostics. 2017 Apr 10;7(6):1633-1649. doi: 10.7150/thno.17588. eCollection 2017. Theranostics. 2017. PMID: 28529641 Free PMC article.
-
Self-assembling asymmetric peptide-dendrimer micelles - a platform for effective and versatile in vitro nucleic acid delivery.Sci Rep. 2018 Mar 19;8(1):4832. doi: 10.1038/s41598-018-22902-9. Sci Rep. 2018. PMID: 29556057 Free PMC article.
References
-
- Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nature reviews. Genetics. 2007;8:173–184. - PubMed
-
- Xie Y, Qiao H, Su Z, Chen M, Ping Q, Sun M. PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials. 2014;35:7978–7991. - PubMed
-
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

