Improvement of Polyunsaturated Fatty Acid Production in Echium acanthocarpum Transformed Hairy Root Cultures by Application of Different Abiotic Stress Conditions
- PMID: 25937970
- PMCID: PMC4393039
- DOI: 10.5402/2013/169510
Improvement of Polyunsaturated Fatty Acid Production in Echium acanthocarpum Transformed Hairy Root Cultures by Application of Different Abiotic Stress Conditions
Abstract
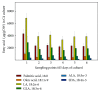
Fatty acids are of great nutritional, therapeutic, and physiological importance, especially the polyunsaturated n-3 fatty acids, possessing larger carbon chains and abundant double bonds or their immediate precursors. A few higher plant species are able to accumulate these compounds, like those belonging to the Echium genus. Here, the novel E. acanthocarpum hairy root system, which is able to accumulate many fatty acids, including stearidonic and α-linolenic acids, was optimized for a better production. The application of abiotic stress resulted in larger yields of stearidonic and α-linolenic acids, 60 and 35%, respectively, with a decrease in linoleic acid, when grown in a nutrient medium consisting of B5 basal salts, sucrose or glucose, and, more importantly, at a temperature of 15°C. The application of osmotic stress employing sorbitol showed no positive influence on the fatty acid yields; furthermore, the combination of a lower culture temperature and glucose did not show a cumulative boosting effect on the yield, although this carbon source was similarly attractive. The abiotic stress also influenced the lipid profile of the cultures, significantly increasing the phosphatidylglycerol fraction but not the total lipid neither their biomass, proving the appropriateness of applying various abiotic stress in this culture to achieve larger yields.
Figures












References
-
- van Gool C. J. A. W., Thijs C., Henquet C. J. M., et al. Gamma-linolenic acid supplementation for prophylaxis of atopic dermatitis–a randomized controlled trial in infants at high familial risk. The American Journal of Clinical Nutrition. 2003;77(4):943–951. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
