Two CGD Families with a Hypomorphic Mutation in the Activation Domain of p67phox
- PMID: 25937994
- PMCID: PMC4414043
Two CGD Families with a Hypomorphic Mutation in the Activation Domain of p67phox
Abstract
Study background: Chronic granulomatous Disease (CGD) is a rare immunodeficiency caused by a defect in the leukocyte NADPH oxidase. This enzyme generates superoxide, which is needed for the killing of bacteria and fungi by phagocytic leukocytes. Most CGD patients have mutations in CYBB, the X-linked gene that encodes gp91phox, the catalytic subunit of the leukocyte NADPH oxidase. We report here three autosomal recessive CGD patients from two families with a homozygous mutation in NCF2, the gene that encodes p67phox, the activator subunit of the NADPH oxidase.
Methods: Leukocyte NADPH oxidase activity, expression of oxidase components and gene sequences were measured with standard methods. The mutation found in the patients' NCF2 gene was expressed as Ala202Val-p67phox in K562 cells to measure its effect on NADPH oxidase activity. Translocation of the mutated p67phox from the cytosol of the patients' neutrophils to the plasma membrane was measured by confocal microscopy and by Western blotting after membrane purification.
Results: The exceptional feature of the A67 CGD patients reported here is that the p.Ala202Val mutation in the activation domain of p67phox was clearly hypomorphic: substantial expression of p67phox protein was noted and the NADPH oxidase activity in the neutrophils of the patients was 20-70% of normal, dependent on the stimulus used to activate the cells. The extent of Ala202Val-p67phox translocation to the plasma membrane during cell activation was also stimulus dependent. Ala202Val-p67phox in K562 cells mediated only about 3% of normal oxidase activity compared to cells transfected with the wild-type p67phox.
Conclusion: The mutation found in NCF2 is the cause of the decreased NADPH oxidase activity and the (mild) clinical problems of the patients. We propose that the p.Ala202Val mutation has changed the conformation of the activation domain of p67phox, resulting in reduced activation of gp91phox.
Keywords: Chronic granulomatous disease; NADPH oxidase; NCF2; hypomorphic mutation; p67phox; p67phox activation domain; p67phox translocation.
Figures
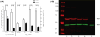

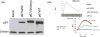
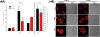
References
-
- Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. - PubMed
-
- Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. - PubMed
-
- Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, et al. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem. 1999;274:25051–25060. - PubMed
-
- Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–215. - PubMed
-
- Roos D, Holland SM, Kuijpers TW. Chronic granulomatous disease. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases A Molecular and Genetic Approach. 3. Oxford University Press; Oxford; 2014. pp. 689–722.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
