Bariatric Surgery Induces Disruption in Inflammatory Signaling Pathways Mediated by Immune Cells in Adipose Tissue: A RNA-Seq Study
- PMID: 25938420
- PMCID: PMC4418598
- DOI: 10.1371/journal.pone.0125718
Bariatric Surgery Induces Disruption in Inflammatory Signaling Pathways Mediated by Immune Cells in Adipose Tissue: A RNA-Seq Study
Abstract
Background: Bariatric surgery is associated to improvements in obesity-associated comorbidities thought to be mediated by a decrease of adipose inflammation. However, the molecular mechanisms behind these beneficial effects are poorly understood.
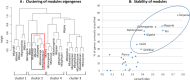
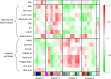
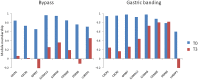
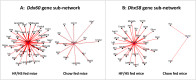
Methodology/principal findings: We analyzed RNA-seq expression profiles in adipose tissue from 22 obese women before and 3 months after surgery. Of 15,972 detected genes, 1214 were differentially expressed after surgery at a 5% false discovery rate. Upregulated genes were mostly involved in the basal cellular machinery. Downregulated genes were enriched in metabolic functions of adipose tissue. At baseline, 26 modules of coexpressed genes were identified. The four most stable modules reflected the innate and adaptive immune responses of adipose tissue. A first module reflecting a non-specific signature of innate immune cells, mainly macrophages, was highly conserved after surgery with the exception of DUSP2 and CD300C. A second module reflected the adaptive immune response elicited by T lymphocytes; after surgery, a disconnection was observed between genes involved in T-cell signaling and mediators of the signal transduction such as CXCR1, CXCR2, GPR97, CCR7 and IL7R. A third module reflected neutrophil-mediated inflammation; after surgery, several genes were dissociated from the module, including S100A8, S100A12, CD300E, VNN2, TUBB1 and FAM65B. We also identified a dense network of 19 genes involved in the interferon-signaling pathway which was strongly preserved after surgery, with the exception of DDX60, an antiviral factor involved in RIG-I-mediated interferon signaling. A similar loss of connection was observed in lean mice compared to their obese counterparts.
Conclusions/significance: These results suggest that improvements of the inflammatory state following surgery might be explained by a disruption of immuno-inflammatory cascades involving a few crucial molecules which could serve as potential therapeutic targets.
Conflict of interest statement
Figures



References
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–781. 10.1016/S0140-6736(14)60460-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

