Antennal Transcriptome Analysis of Odorant Reception Genes in the Red Turpentine Beetle (RTB), Dendroctonus valens
- PMID: 25938508
- PMCID: PMC4418697
- DOI: 10.1371/journal.pone.0125159
Antennal Transcriptome Analysis of Odorant Reception Genes in the Red Turpentine Beetle (RTB), Dendroctonus valens
Abstract
Background: The red turpentine beetle (RTB), Dendroctonus valens LeConte (Coleoptera: Curculionidae, Scolytinae), is a destructive invasive pest of conifers which has become the second most important forest pest nationwide in China. Dendroctonus valens is known to use host odors and aggregation pheromones, as well as non-host volatiles, in host location and mass-attack modulation, and thus antennal olfaction is of the utmost importance for the beetles' survival and fitness. However, information on the genes underlying olfaction has been lacking in D. valens. Here, we report the antennal transcriptome of D. valens from next-generation sequencing, with the goal of identifying the olfaction gene repertoire that is involved in D. valens odor-processing.
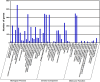
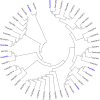
Results: We obtained 51 million reads that were assembled into 61,889 genes, including 39,831 contigs and 22,058 unigenes. In total, we identified 68 novel putative odorant reception genes, including 21 transcripts encoding for putative odorant binding proteins (OBP), six chemosensory proteins (CSP), four sensory neuron membrane proteins (SNMP), 22 odorant receptors (OR), four gustatory receptors (GR), three ionotropic receptors (IR), and eight ionotropic glutamate receptors. We also identified 155 odorant/xenobiotic degradation enzymes from the antennal transcriptome, putatively identified to be involved in olfaction processes including cytochrome P450s, glutathione-S-transferases, and aldehyde dehydrogenase. Predicted protein sequences were compared with counterparts in Tribolium castaneum, Megacyllene caryae, Ips typographus, Dendroctonus ponderosae, and Agrilus planipennis.
Conclusion: The antennal transcriptome described here represents the first study of the repertoire of odor processing genes in D. valens. The genes reported here provide a significant addition to the pool of identified olfactory genes in Coleoptera, which might represent novel targets for insect management. The results from our study also will assist with evolutionary analyses of coleopteran olfaction.
Conflict of interest statement
Figures







References
-
- Andersson MN, Grosse-Wilde E, Keeling CI, Bengtsson JM, Yuen MM, Li M, et al. (2013) Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae (Coleoptera: Curculionidae: Scolytinae). BMC Genomics 14: 198 10.1186/1471-2164-14-198 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

