A High Throughput Protein Microarray Approach to Classify HIV Monoclonal Antibodies and Variant Antigens
- PMID: 25938510
- PMCID: PMC4418728
- DOI: 10.1371/journal.pone.0125581
A High Throughput Protein Microarray Approach to Classify HIV Monoclonal Antibodies and Variant Antigens
Abstract
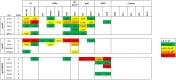
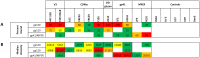
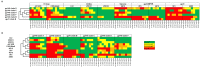
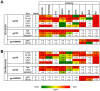
In recent years, high throughput discovery of human recombinant monoclonal antibodies (mAbs) has been applied to greatly advance our understanding of the specificity, and functional activity of antibodies against HIV. Thousands of antibodies have been generated and screened in functional neutralization assays, and antibodies associated with cross-strain neutralization and passive protection in primates, have been identified. To facilitate this type of discovery, a high throughput-screening tool is needed to accurately classify mAbs, and their antigen targets. In this study, we analyzed and evaluated a prototype microarray chip comprised of the HIV-1 recombinant proteins gp140, gp120, gp41, and several membrane proximal external region peptides. The protein microarray analysis of 11 HIV-1 envelope-specific mAbs revealed diverse binding affinities and specificities across clades. Half maximal effective concentrations, generated by our chip analysis, correlated significantly (P<0.0001) with concentrations from ELISA binding measurements. Polyclonal immune responses in plasma samples from HIV-1 infected subjects exhibited different binding patterns, and reactivity against printed proteins. Examining the totality of the specificity of the humoral response in this way reveals the exquisite diversity, and specificity of the humoral response to HIV.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

