PARP inhibitor ABT-888 affects response of MDA-MB-231 cells to doxorubicin treatment, targeting Snail expression
- PMID: 25938539
- PMCID: PMC4558132
- DOI: 10.18632/oncotarget.3634
PARP inhibitor ABT-888 affects response of MDA-MB-231 cells to doxorubicin treatment, targeting Snail expression
Abstract
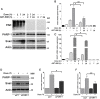
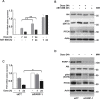
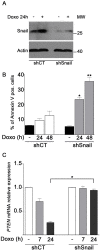
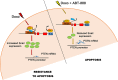
To overcome cancer cells resistance to pharmacological therapy, the development of new therapeutic approaches becomes urgent. For this purpose, the use of poly(ADP-ribose) polymerase (PARP) inhibitors in combination with other cytotoxic agents could represent an efficacious strategy. Poly(ADP-ribosyl)ation (PARylation) is a post-translational modification that plays a well characterized role in the cellular decisions of life and death. Recent findings indicate that PARP-1 may control the expression of Snail, the master gene of epithelial-mesenchymal transition (EMT). Snail is highly represented in different resistant tumors, functioning as a factor regulating anti-apoptotic programmes. MDA-MB-231 is a Snail-expressing metastatic breast cancer cell line, which exhibits chemoresistance properties when treated with damaging agents. In this study, we show that the PARP inhibitor ABT-888 was capable to modulate the MDA-MB-231 cell response to doxorubicin, leading to an increase in the rate of apoptosis. Our further results indicate that PARP-1 controlled Snail expression at transcriptional level in cells exposed to doxorubicin. Given the increasing interest in the employment of PARP inhibitors as chemotherapeutic adjuvants, our in vitro results suggest that one of the mechanisms through which PARP inhibition can chemosensitize cancer cells in vivo, is targeting Snail expression thus promoting apoptosis.
Keywords: PARP inhibitors; Snail; breast cancer; chemoresistance.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochemical pharmacology. 2013;85:1219–1226. - PubMed
-
- Videira M, Reis RL, Brito MA. Deconstructing breast cancer cell biology and the mechanisms of multidrug resistance. Biochimica et biophysica acta. 2014;1846:312–325. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

