Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes
- PMID: 25938585
- PMCID: PMC4418673
- DOI: 10.1371/journal.pone.0126194
Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes
Erratum in
-
Correction: nicotinamide impairs entry into and exit from meiosis I in mouse oocytes.PLoS One. 2015 Jun 3;10(6):e0130058. doi: 10.1371/journal.pone.0130058. eCollection 2015. PLoS One. 2015. PMID: 26038838 Free PMC article. No abstract available.
Abstract
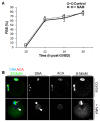
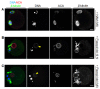
Following exit from meiosis I, mammalian oocytes immediately enter meiosis II without an intervening interphase, accompanied by rapid reassembly of a bipolar spindle that maintains condensed chromosomes in a metaphase configuration (metaphase II arrest). Here we study the effect of nicotinamide (NAM), a non-competitive pan-sirtuin inhibitor, during meiotic maturation in mouse oocytes. Sirtuins are a family of seven NAD+-dependent deacetylases (Sirt1-7), which are involved in multiple cellular processes and are emerging as important regulators in oocytes and embryos. We found that NAM significantly delayed entry into meiosis I associated with delayed accumulation of the Cdk1 co-activator, cyclin B1. GVBD was also inhibited by the Sirt2-specific inhibitor, AGK2, and in a very similar pattern to NAM, supporting the notion that as in somatic cells, NAM inhibits sirtuins in oocytes. NAM did not affect subsequent spindle assembly, chromosome alignment or the timing of first polar body extrusion (PBE). Unexpectedly, however, in the majority of oocytes with a polar body, chromatin was decondensed and a nuclear structure was present. An identical phenotype was observed when flavopiridol was used to induce Cdk1 inactivation during late meiosis I prior to PBE, but not if Cdk1 was inactivated after PBE when metaphase II arrest was already established, altogether indicating that NAM impaired establishment rather than maintenance of metaphase II arrest. During meiosis I exit in NAM-treated medium, we found that cyclin B1 levels were lower and inhibitory Cdk1 phosphorylation was increased compared with controls. Although activation of the anaphase-promoting complex-Cdc20 (APC-Cdc20) occurred on-time in NAM-treated oocytes, Cdc20 levels were higher in very late meiosis I, pointing to exaggerated APC-Cdc20-mediated proteolysis as a reason for lower cyclin B1 levels. Collectively, therefore, our data indicate that by disrupting Cdk1 regulation, NAM impairs entry into meiosis I and the establishment of metaphase II arrest.
Conflict of interest statement
Figures





References
-
- Homer H. Getting into and out of oocyte maturation In: Trounson A, Gosden R, Eichenlaub-Ritter U, editors. Biology and Pathology of the Oocyte. 2nd ed United Kingdom: Cambridge University Press; 2013. pp. 119–41.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

