High Pressure Homogenization of Porcine Pepsin Protease: Effects on Enzyme Activity, Stability, Milk Coagulation Profile and Gel Development
- PMID: 25938823
- PMCID: PMC4418662
- DOI: 10.1371/journal.pone.0125061
High Pressure Homogenization of Porcine Pepsin Protease: Effects on Enzyme Activity, Stability, Milk Coagulation Profile and Gel Development
Abstract
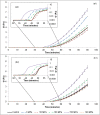
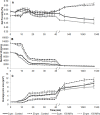
This study investigated the effect of high pressure homogenization (HPH) (up to 190 MPa) on porcine pepsin (proteolytic and milk-clotting activities), and the consequences of using the processed enzyme in milk coagulation and gel formation (rheological profile, proteolysis, syneresis, and microstructure). Although the proteolytic activity (PA) was not altered immediately after the HPH process, it reduced during enzyme storage, with a 5% decrease after 60 days of storage for samples obtained with the enzyme processed at 50, 100 and 150 MPa. HPH increased the milk-clotting activity (MCA) of the enzyme processed at 150 MPa, being 15% higher than the MCA of non-processed samples after 60 days of storage. The enzyme processed at 150 MPa produced faster aggregation and a more consistent milk gel (G' value 92% higher after 90 minutes) when compared with the non-processed enzyme. In addition, the gels produced with the enzyme processed at 150 MPa showed greater syneresis after 40 minutes of coagulation (forming a more compact protein network) and lower porosity (evidenced by confocal microscopy). These effects on the milk gel can be associated with the increment in MCA and reduction in PA caused by the effects of HPH on pepsin during storage. According to the results, HPH stands out as a process capable of changing the proteolytic characteristics of porcine pepsin, with improvements on the milk coagulation step and gel characteristics. Therefore, the porcine pepsin submitted to HPH process can be a suitable alternative for the production of cheese.
Conflict of interest statement
Figures





Similar articles
-
Manufacture of acid gels from skim milk using high-pressure homogenization.J Dairy Sci. 2008 Oct;91(10):3761-7. doi: 10.3168/jds.2008-1321. J Dairy Sci. 2008. PMID: 18832197
-
Effect of high-pressure homogenisation on rheological properties of rennet-induced skim milk and standardised milk gels.J Dairy Res. 2009 Aug;76(3):294-300. doi: 10.1017/S0022029909004117. Epub 2009 May 18. J Dairy Res. 2009. PMID: 19445828
-
Biophysical evaluation of milk-clotting enzymes processed by high pressure.Food Res Int. 2017 Jul;97:116-122. doi: 10.1016/j.foodres.2017.03.042. Epub 2017 Mar 27. Food Res Int. 2017. PMID: 28578031
-
Activation of porcine pepsinogen A. The stability of two non-covalent activation intermediates at pH 8.5.Eur J Biochem. 1993 Oct 1;217(1):137-42. doi: 10.1111/j.1432-1033.1993.tb18228.x. Eur J Biochem. 1993. PMID: 8223551
-
Modification of enzymes by use of high-pressure homogenization.Food Res Int. 2018 Jul;109:120-125. doi: 10.1016/j.foodres.2018.04.011. Epub 2018 Apr 14. Food Res Int. 2018. PMID: 29803433 Review.
Cited by
-
Benefits of Camel Milk over Cow and Goat Milk for Infant and Adult Health in Fighting Chronic Diseases: A Review.Nutrients. 2024 Nov 10;16(22):3848. doi: 10.3390/nu16223848. Nutrients. 2024. PMID: 39599634 Free PMC article. Review.
-
Composition, Structure, and Digestive Dynamics of Milk From Different Species-A Review.Front Nutr. 2020 Oct 6;7:577759. doi: 10.3389/fnut.2020.577759. eCollection 2020. Front Nutr. 2020. PMID: 33123547 Free PMC article. Review.
References
-
- Euromonitor International—Related Analysis. 2012. Cheese in Brazil. Available: http://www.portal.euromonitor.com/Portal/Pages/Statistics/Statistics.aspx. Accessed 2014 Jun 18.
-
- Food Agriculture Organization. 2010. Milk and Milk Products. Available: http://faostat.fao.org/site/610/default.aspx#ancor. Accessed 2013 Aug 10.
-
- Fox PF, Kelly AL. The caseins In: Yada RY, editor. Proteins in Food Processing. Boca Raton, FL: CRC Press; 2004. pp. 29–71.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

