The miRNA Transcriptome Directly Reflects the Physiological and Biochemical Differences between Red, White, and Intermediate Muscle Fiber Types
- PMID: 25938964
- PMCID: PMC4463610
- DOI: 10.3390/ijms16059635
The miRNA Transcriptome Directly Reflects the Physiological and Biochemical Differences between Red, White, and Intermediate Muscle Fiber Types
Abstract
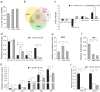
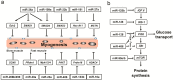
MicroRNAs (miRNAs) are small non-coding RNAs that can regulate their target genes at the post-transcriptional level. Skeletal muscle comprises different fiber types that can be broadly classified as red, intermediate, and white. Recently, a set of miRNAs was found expressed in a fiber type-specific manner in red and white fiber types. However, an in-depth analysis of the miRNA transcriptome differences between all three fiber types has not been undertaken. Herein, we collected 15 porcine skeletal muscles from different anatomical locations, which were then clearly divided into red, white, and intermediate fiber type based on the ratios of myosin heavy chain isoforms. We further illustrated that three muscles, which typically represented each muscle fiber type (i.e., red: peroneal longus (PL), intermediate: psoas major muscle (PMM), white: longissimus dorsi muscle (LDM)), have distinct metabolic patterns of mitochondrial and glycolytic enzyme levels. Furthermore, we constructed small RNA libraries for PL, PMM, and LDM using a deep sequencing approach. Results showed that the differentially expressed miRNAs were mainly enriched in PL and played a vital role in myogenesis and energy metabolism. Overall, this comprehensive analysis will contribute to a better understanding of the miRNA regulatory mechanism that achieves the phenotypic diversity of skeletal muscles.
Figures

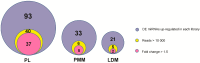
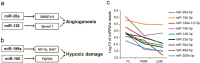
References
-
- Choi Y., Kim B. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009;122:105–118. doi: 10.1016/j.livsci.2008.08.015. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

