Changing the face of kynurenines and neurotoxicity: therapeutic considerations
- PMID: 25938971
- PMCID: PMC4463617
- DOI: 10.3390/ijms16059772
Changing the face of kynurenines and neurotoxicity: therapeutic considerations
Abstract
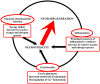
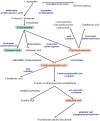
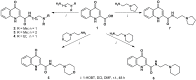
Kynurenines are the products of tryptophan metabolism. Among them, kynurenine and kynurenic acid are generally thought to have neuroprotective properties, while 3-hydroxykynurenine, 3-hydroxyanthranilic acid and quinolinic acid are considered neurotoxic. They participate in immunoregulation and inflammation and possess pro- or anti-excitotoxic properties, and their involvement in oxidative stress has also been suggested. Consequently, it is not surprising that kynurenines have been closely related to neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and multiple sclerosis. More information about the less-known metabolites, picolinic and cinnabarinic acid, evaluation of new receptorial targets, such as aryl-hydrocarbon receptors, and intensive research on the field of the immunomodulatory function of kynurenines delineated the high importance of this pathway in general homeostasis. Emerging knowledge about the kynurenine pathway provides new target points for the development of therapeutical solutions against neurodegenerative diseases.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

