The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2
- PMID: 25939035
- PMCID: PMC4446599
- DOI: 10.3390/md13052666
The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2
Abstract
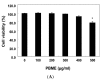
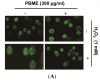
This study was designed to examine the protective effects of the marine brown algae Petalonia binghamiae against oxidative stress-induced cellular damage and to elucidate the underlying mechanisms. P. binghamiae methanol extract (PBME) prevented hydrogen peroxide (H2O2)-induced growth inhibition and exhibited scavenging activity against intracellular reactive oxygen species (ROS) induced by H2O2 in mouse-derived C2C12 myoblasts. PBME also significantly attenuated H2O2-induced comet tail formation in a comet assay, histone γH2A.X phosphorylation, and annexin V-positive cells, suggesting that PBME prevented H2O2-induced cellular DNA damage and apoptotic cell death. Furthermore, PBME increased the levels of heme oxygenase-1 (HO-1), a potent antioxidant enzyme, associated with the induction of nuclear factor-erythroid 2 related factor 2 (Nrf2). However, zinc protoporphyrin IX, a HO-1 competitive inhibitor, significantly abolished the protective effects of PBME on H2O2-induced ROS generation, growth inhibition, and apoptosis. Collectively, these results demonstrate that PBME augments the antioxidant defense capacity through activation of the Nrf2/HO-1 pathway.
Figures






References
-
- Vesely M.J., Exon D.J., Clark J.E., Foresti R., Green C.J., Motterlini R. Heme oxygenase-1 induction in skeletal muscle cells: Hemin and sodium nitroprusside are regulators in vitro. Am. J. Physiol. 1998;275:C1087–C1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

