Elucidating steroid alkaloid biosynthesis in Veratrum californicum: production of verazine in Sf9 cells
- PMID: 25939370
- PMCID: PMC4464957
- DOI: 10.1111/tpj.12871
Elucidating steroid alkaloid biosynthesis in Veratrum californicum: production of verazine in Sf9 cells
Abstract
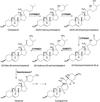
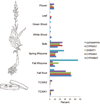
Steroid alkaloids have been shown to elicit a wide range of pharmacological effects that include anticancer and antifungal activities. Understanding the biosynthesis of these molecules is essential to bioengineering for sustainable production. Herein, we investigate the biosynthetic pathway to cyclopamine, a steroid alkaloid that shows promising antineoplastic activities. Supply of cyclopamine is limited, as the current source is solely derived from wild collection of the plant Veratrum californicum. To elucidate the early stages of the pathway to cyclopamine, we interrogated a V. californicum RNA-seq dataset using the cyclopamine accumulation profile as a predefined model for gene expression with the pattern-matching algorithm Haystack. Refactoring candidate genes in Sf9 insect cells led to discovery of four enzymes that catalyze the first six steps in steroid alkaloid biosynthesis to produce verazine, a predicted precursor to cyclopamine. Three of the enzymes are cytochromes P450 while the fourth is a γ-aminobutyrate transaminase; together they produce verazine from cholesterol.
Keywords: California corn lily; Haystack; Veratrum californicum; cyclopamine; steroid alkaloids; verazine.
© 2015 The Authors The Plant Journal © 2015 John Wiley & Sons Ltd.
Figures



References
-
- Adam G, Schreiber K, Tomko J, Vassova A. Verazine, a new Veratrum alkaloid with 22, 26-imino-cholestan structure. Tetrahedron. 1967;23:167–171. - PubMed
-
- Bahra M, Kamphues C, Boas-Knoop S, Lippert S, Esendik U, Schuller U, Hartmann W, Waha A, Neuhaus P, Heppner F, Pietsch T, Koch A. Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas. Pancreas. 2012;41:222–229. - PubMed
-
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

