Precise Three-Dimensional Scan-Free Multiple-Particle Tracking over Large Axial Ranges with Tetrapod Point Spread Functions
- PMID: 25939423
- PMCID: PMC4462996
- DOI: 10.1021/acs.nanolett.5b01396
Precise Three-Dimensional Scan-Free Multiple-Particle Tracking over Large Axial Ranges with Tetrapod Point Spread Functions
Abstract
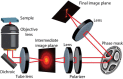
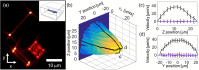
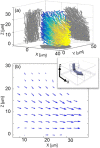
We employ a novel framework for information-optimal microscopy to design a family of point spread functions (PSFs), the Tetrapod PSFs, which enable high-precision localization of nanoscale emitters in three dimensions over customizable axial (z) ranges of up to 20 μm with a high numerical aperture objective lens. To illustrate, we perform flow profiling in a microfluidic channel and show scan-free tracking of single quantum-dot-labeled phospholipid molecules on the surface of living, thick mammalian cells.
Keywords: 3D imaging; PSF engineering; nanoscopy; single particle tracking; single-molecule imaging; super-resolution microscopy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

