Evaluating an extended rehabilitation service for stroke patients (EXTRAS): study protocol for a randomised controlled trial
- PMID: 25939584
- PMCID: PMC4445280
- DOI: 10.1186/s13063-015-0704-3
Evaluating an extended rehabilitation service for stroke patients (EXTRAS): study protocol for a randomised controlled trial
Abstract
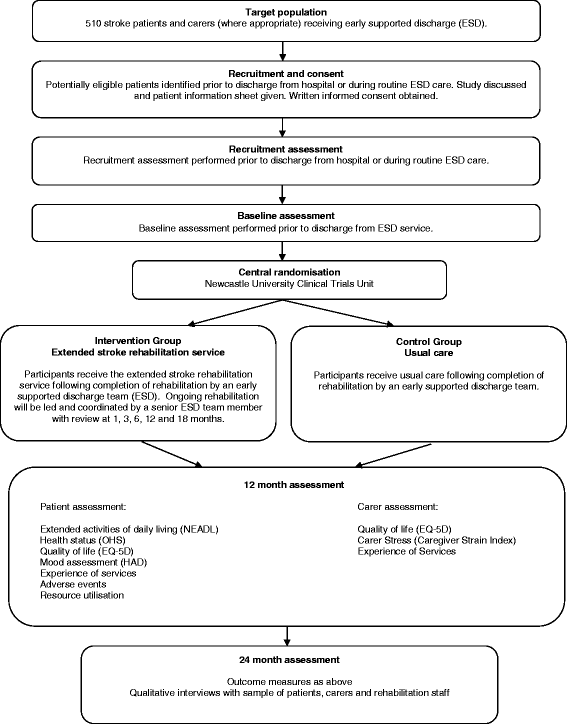
Background: Development of longer term stroke rehabilitation services is limited by lack of evidence of effectiveness for specific interventions and service models. We describe the protocol for a multicentre randomised controlled trial which is evaluating an extended stroke rehabilitation service. The extended service commences when routine 'organised stroke care' (stroke unit and early supported discharge (ESD)) ends.
Methods/design: This study is a multicentre randomised controlled trial with health economic and process evaluations. It is set within NHS stroke services which provide ESD. Participants are adults who have experienced a new stroke (and carer if appropriate), discharged from hospital under the care of an ESD team. The intervention group receives an extended stroke rehabilitation service provided for 18 months following completion of ESD. The extended rehabilitation service involves regular contact with a senior ESD team member who leads and coordinates further rehabilitation. Contact is usually by telephone. The control group receives usual stroke care post-ESD. Usual care may involve referral of patients to a range of rehabilitation services upon completion of ESD in accordance with local clinical practice. Randomisation is via a central independent web-based service. The primary outcome is extended activities of daily living (Nottingham Extended Activities of Daily Living Scale) at 24 months post-randomisation. Secondary outcomes (at 12 and 24 months post-randomisation) are health status, quality of life, mood and experience of services for patients, and quality of life, experience of services and carer stress for carers. Resource use and adverse events are also collected. Outcomes are undertaken by a blinded assessor. Implementation and delivery of the extended stroke rehabilitation service will also be described. Semi-structured interviews will be conducted with a subsample of participants and staff to gain insight into perceptions and experiences of rehabilitation services delivered or received. Allowing for 25% attrition, 510 participants are needed to provide 90% power to detect a difference in mean Nottingham Extended Activities of Daily Living Scale score of 6 with a 5% significance level.
Discussion: The provision of longer term support for stroke survivors is currently limited. The results from this trial will inform future stroke service planning and configuration.
Trial registration: This trial was registered with ISRCTN (identifier: ISRCTN45203373 ) on 9 August 2012.
Figures
References
-
- National Audit Office . Reducing brain damage: faster access to better stroke care. London: National Audit Office; 2005.
-
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Wolfe C. UK stroke survivor needs survey. London: The Stroke Association; 2010.
-
- Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev. 2012:CD000443. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous