ZFP36 stabilizes RIP1 via degradation of XIAP and cIAP2 thereby promoting ripoptosome assembly
- PMID: 25939870
- PMCID: PMC4424499
- DOI: 10.1186/s12885-015-1388-5
ZFP36 stabilizes RIP1 via degradation of XIAP and cIAP2 thereby promoting ripoptosome assembly
Abstract
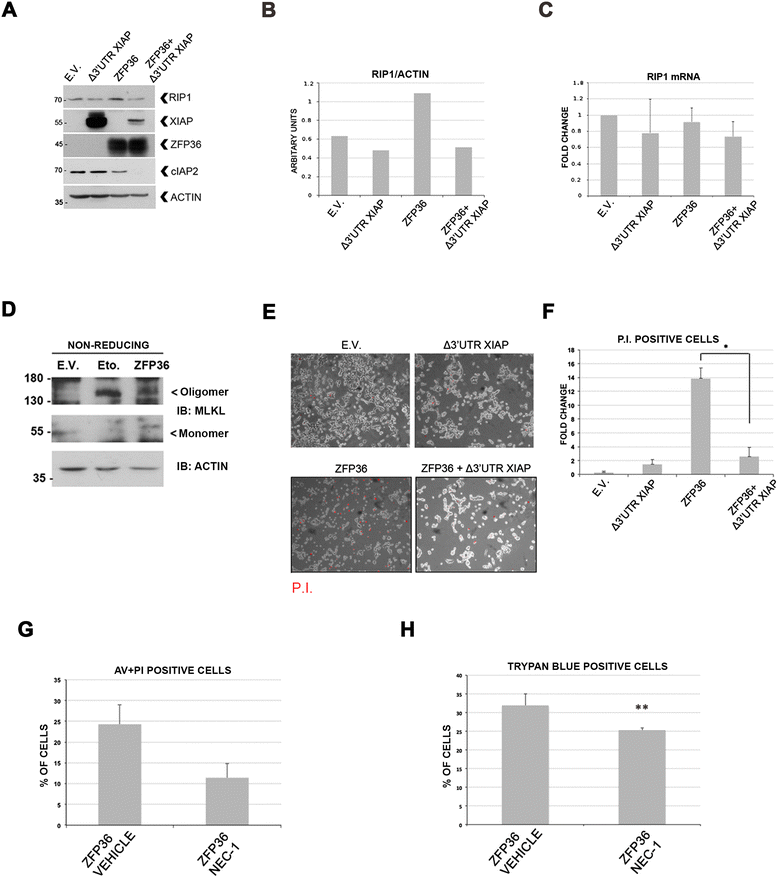
Background: ZFP36 is an mRNA binding protein that exerts anti-tumor activity in glioblastoma by triggering cell death, associated to an increase in the stability of the kinase RIP1.
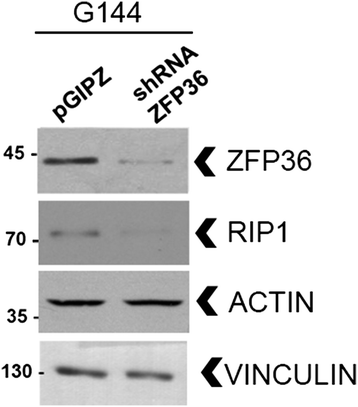
Methods: We used cell death assays, size exclusion chromatography, Co-Immunoprecipitation, shRNA lentivectors and glioma neural stem cells to determine the effects of ZFP36 on the assembly of a death complex containing RIP1 and on the induction of necroptosis.
Results: Here we demonstrate that ZFP36 promotes the assembly of the death complex called Ripoptosome and induces RIP1-dependent death. This involves the depletion of the ubiquitine ligases cIAP2 and XIAP and leads to the association of RIP1 to caspase-8 and FADD. Moreover, we show that ZFP36 controls RIP1 levels in glioma neural stem cell lines.
Conclusions: We provide a molecular mechanism for the tumor suppressor role of ZFP36, and the first evidence for Ripoptosome assembly following ZFP36 expression. These findings suggest that ZFP36 plays an important role in RIP1-dependent cell death in conditions where IAPs are depleted.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

