Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs
- PMID: 25939885
- PMCID: PMC7117181
- DOI: 10.1016/j.vetmic.2015.04.022
Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs
Abstract
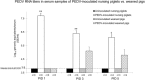
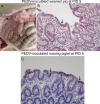
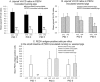
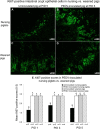
Our study demonstrated potential mechanisms by which porcine epidemic diarrhea virus (PEDV) infection induces greater disease severity of nursing vs. weaned conventional pigs. Twenty-six-day-old weaned [PEDV-inoculated (n=11); mock (n=9)] and 9-day-old nursing pigs [PEDV-inoculated (n=9); mock (n=11)] were inoculated orally [8.9 log10 genomic equivalents (GE)/pig] with PC21A strain or mock (MEM). Pigs were monitored for clinical signs and PEDV RNA titers in feces and serum. For pathology and immunofluorescence staining for Ki67 (marker for crypt proliferation) and LGR5 (marker for crypt stem cell), 3-4 pigs were euthanized at postinoculation days (PIDs) 1, 3 and 5. Severe watery diarrhea and atrophic enteritis with moderate to high PEDV RNA titers in feces (7.5-12.2 log10 GE/ml) and low viral RNA titers in serum (5.6-8.6 log10 GE/ml) were observed in all inoculated nursing piglets at PIDs 1-5. In contrast, weaned pigs did not show evidence of PEDV infection at PID 1. Pigs exhibited high fecal shedding titers at PIDs 2-5 and mild to severe atrophic enteritis at PIDs 3-5, indicating a longer incubation for PEDV infection. While uninoculated or inoculated 27-31-day-old pigs showed large numbers of Ki67- or LGR5-positive cells in the intestinal crypts, there was a lack of LGR5-positive cells and low proliferation of crypts in jejunum of uninoculated 10-14-day-old piglets, possibly causing a slower turnover of enterocytes; however, the number of LGR5-positive cells and proliferation of intestinal crypts increased remarkably at 3-5 days after inoculation. Biologic mediators that promote crypt stem cell regeneration would be targets to improve the intestinal epithelium renewal during PEDV infection.
Keywords: PEDV; Pathogenesis; Pig; Porcine epidemic diarrhea virus; Virus.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
-
- Burkey T.E., Skjolaas K.A., Minton J.E. Board-invited review: porcine mucosal immunity of the gastrointestinal tract. J. Anim. Sci. 2009;87:1493–1501. - PubMed
-
- Chung W.B., Chan W.H., Chaung H.C., Lien Y., Wu C.C., Huang Y.L. Real-time PCR for quantitation of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 in naturally-infected and challenged pigs. J. Virol. Methods. 2005;124:11–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

