Treatment of patients with diabetic peripheral neuropathic pain in China: a double-blind randomised trial of duloxetine vs. placebo
- PMID: 25939897
- PMCID: PMC4682474
- DOI: 10.1111/ijcp.12641
Treatment of patients with diabetic peripheral neuropathic pain in China: a double-blind randomised trial of duloxetine vs. placebo
Abstract
Background: Duloxetine has been approved in the United States, European Union and some Asian countries for the treatment of diabetic peripheral neuropathic pain (DPNP). We assessed the efficacy and safety of duloxetine (60 mg once daily) compared with placebo in Chinese patients suffering from DPNP.
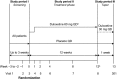
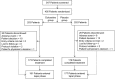
Methods: This was a phase 3, multicenter, randomised, double-blind, parallel, placebo-controlled, 12-week trial of the treatment of DPNP with duloxetine. Subjects were male and female outpatients ≥ 18 years of age with DPNP, as assessed by the Michigan Neuropathy Screening Instrument, and had a rating of ≥ 4 on the Brief Pain Inventory-Modified Short Form-Severity weekly average pain item. The primary efficacy measure was the reduction in pain severity from baseline to 12 weeks, as measured by the weekly mean of 24-h average pain ratings recorded in the patient's diary. Mean changes from baseline in efficacy measures were analysed by a restricted maximum likelihood-based, mixed-effects model repeated measures approach and by analysis of covariance.
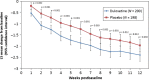
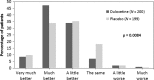
Results: Of the 405 patients randomised, 203 patients were assigned to duloxetine 60 mg once daily and 202 patients were assigned to placebo. Duloxetine-treated patients showed significantly greater pain relief on 24-h average pain ratings compared with placebo-treated patients each week of the 12-week study period [week 12: least squares (LS) mean change duloxetine: -2.40, placebo: -1.97; LS mean change difference (95% confidence interval) = -0.43 (-0.82, -0.04), p = 0.030]. Compared with placebo, patients treated with duloxetine experienced higher rates of nausea (p = 0.010), somnolence (p < 0.001) and asthenia (p = 0.002).
Conclusions: Duloxetine-treated patients showed significantly greater pain relief compared with placebo-treated patients over the 12-week study period. Duloxetine was shown in Chinese patients to have a safety profile similar to that found in previous duloxetine trials.
Trial registration: ClinicalTrials.gov NCT01179672.
© 2015 Eli Lily and Company. The International Journal of Clinical Practice published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials.Syst Rev. 2023 Mar 21;12(1):53. doi: 10.1186/s13643-023-02185-6. Syst Rev. 2023. PMID: 36945033 Free PMC article.
-
Efficacy and safety of duloxetine versus placebo in adolescents with juvenile fibromyalgia: results from a randomized controlled trial.Pediatr Rheumatol Online J. 2019 May 28;17(1):27. doi: 10.1186/s12969-019-0325-6. Pediatr Rheumatol Online J. 2019. PMID: 31138224 Free PMC article. Clinical Trial.
-
A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain.Neurology. 2006 Oct 24;67(8):1411-20. doi: 10.1212/01.wnl.0000240225.04000.1a. Neurology. 2006. PMID: 17060567 Clinical Trial.
-
Duloxetine for the management of diabetic peripheral neuropathic pain: response profile.Pain Med. 2007 Jul-Aug;8(5):397-409. doi: 10.1111/j.1526-4637.2007.00305.x. Pain Med. 2007. PMID: 17661853 Clinical Trial.
-
Efficacy and safety of duloxetine in osteoarthritis or chronic low back pain: a Systematic review and meta-analysis.Osteoarthritis Cartilage. 2020 Jun;28(6):721-734. doi: 10.1016/j.joca.2020.03.001. Epub 2020 Mar 10. Osteoarthritis Cartilage. 2020. PMID: 32169731
Cited by
-
Different Drugs for the Treatment of Painful Diabetic Peripheral Neuropathy: A Meta-Analysis.Front Neurol. 2021 Oct 29;12:682244. doi: 10.3389/fneur.2021.682244. eCollection 2021. Front Neurol. 2021. PMID: 34777192 Free PMC article.
-
Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials.Syst Rev. 2023 Mar 21;12(1):53. doi: 10.1186/s13643-023-02185-6. Syst Rev. 2023. PMID: 36945033 Free PMC article.
-
The short-term effect and safety of duloxetine in osteoarthritis: A systematic review and meta-analysis.Medicine (Baltimore). 2019 Nov;98(44):e17541. doi: 10.1097/MD.0000000000017541. Medicine (Baltimore). 2019. PMID: 31689755 Free PMC article.
-
A Systematic Review of Efficacy, Safety, and Tolerability of Duloxetine.Front Psychiatry. 2020 Oct 23;11:554899. doi: 10.3389/fpsyt.2020.554899. eCollection 2020. Front Psychiatry. 2020. PMID: 33192668 Free PMC article.
-
Non-opioid psychiatric medications for chronic pain: systematic review and meta-analysis.Front Pain Res (Lausanne). 2024 Oct 10;5:1398442. doi: 10.3389/fpain.2024.1398442. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 39449766 Free PMC article.
References
-
- Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–9. - PubMed
-
- Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14. - PubMed
-
- Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–8. - PubMed
-
- Zuo H, Shi Z, Hussain A. Prevalence, trends and risk factors for the diabetes epidemic in China: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;104:63–72. - PubMed
-
- Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
