Parvulin 17-catalyzed Tubulin Polymerization Is Regulated by Calmodulin in a Calcium-dependent Manner
- PMID: 25940090
- PMCID: PMC4505421
- DOI: 10.1074/jbc.M114.593228
Parvulin 17-catalyzed Tubulin Polymerization Is Regulated by Calmodulin in a Calcium-dependent Manner
Abstract
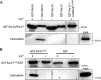
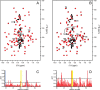
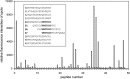
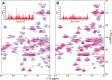
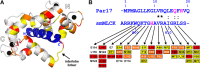
Recently we have shown that the peptidyl-prolyl cis/trans isomerase parvulin 17 (Par17) interacts with tubulin in a GTP-dependent manner, thereby promoting the formation of microtubules. Microtubule assembly is regulated by Ca(2+)-loaded calmodulin (Ca(2+)/CaM) both in the intact cell and under in vitro conditions via direct interaction with microtubule-associated proteins. Here we provide the first evidence that Ca(2+)/CaM interacts also with Par17 in a physiologically relevant way, thus preventing Par17-promoted microtubule assembly. In contrast, parvulin 14 (Par14), which lacks only the first 25 N-terminal residues of the Par17 sequence, does not interact with Ca(2+)/CaM, indicating that this interaction is exclusive for Par17. Pulldown experiments and chemical shift perturbation analysis with (15)N-labeled Par17 furthermore confirmed that calmodulin (CaM) interacts in a Ca(2+)-dependent manner with the Par17 N terminus. The reverse experiment with (15)N-labeled Ca(2+)/CaM demonstrated that the N-terminal Par17 segment binds to both CaM lobes simultaneously, indicating that Ca(2+)/CaM undergoes a conformational change to form a binding channel between its two lobes, apparently similar to the structure of the CaM-smMLCK(796-815) complex. In vitro tubulin polymerization assays furthermore showed that Ca(2+)/CaM completely suppresses Par17-promoted microtubule assembly. The results imply that Ca(2+)/CaM binding to the N-terminal segment of Par17 causes steric hindrance of the Par17 active site, thus interfering with the Par17/tubulin interaction. This Ca(2+)/CaM-mediated control of Par17-assisted microtubule assembly may provide a mechanism that couples Ca(2+) signaling with microtubule function.
Keywords: calcium; calmodulin (CaM); chemical shift perturbation (CSP) analysis; microtubule-associated protein (MAP); nuclear magnetic resonance (NMR); parvulin; protein conformation; protein-protein interaction; tubulin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Galat A., Metcalfe S. M. (1995) Peptidylproline cis/trans isomerases. Prog. Biophys. Mol. Biol. 63, 67–118 - PubMed
-
- Fanghänel J., Fischer G. (2004) Insights into the catalytic mechanism of peptidyl prolyl cis/trans isomerases. Front. Biosci. 9, 3453–3478 - PubMed
-
- Yaffe M. B., Schutkowski M., Shen M., Zhou X. Z., Stukenberg P. T., Rahfeld J. U., Xu J., Kuang J., Kirschner M. W., Fischer G., Cantley L. C., Lu K. P. (1997) Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278, 1957–1960 - PubMed
-
- Lu K. P. (2003) Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell 4, 175–180 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

