Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes
- PMID: 25940348
- PMCID: PMC4427784
- DOI: 10.1083/jcb.201412046
Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes
Abstract
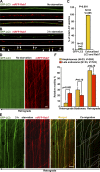
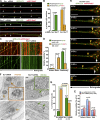
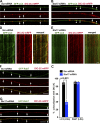
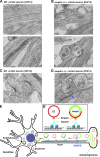
Efficient degradation of autophagic vacuoles (AVs) via lysosomes is an important cellular homeostatic process. This is particularly challenging for neurons because mature acidic lysosomes are relatively enriched in the soma. Although dynein-driven retrograde transport of AVs was suggested, a fundamental question remains how autophagosomes generated at distal axons acquire dynein motors for retrograde transport toward the soma. In this paper, we demonstrate that late endosome (LE)-loaded dynein-snapin complexes drive AV retrograde transport in axons upon fusion of autophagosomes with LEs into amphisomes. Blocking the fusion with syntaxin17 knockdown reduced recruitment of dynein motors to AVs, thus immobilizing them in axons. Deficiency in dynein-snapin coupling impaired AV transport ,: resulting in AV accumulation in neurites and synaptic terminals. Altogether, our study provides the first evidence that autophagosomes recruit dynein through fusion with LEs and reveals a new motor-adaptor sharing mechanism by which neurons may remove distal AVs engulfing aggregated proteins and dysfunctional organelles for efficient degradation in the soma.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

