The SOX9 upstream region prone to chromosomal aberrations causing campomelic dysplasia contains multiple cartilage enhancers
- PMID: 25940622
- PMCID: PMC4477657
- DOI: 10.1093/nar/gkv426
The SOX9 upstream region prone to chromosomal aberrations causing campomelic dysplasia contains multiple cartilage enhancers
Abstract
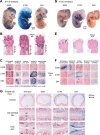
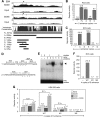
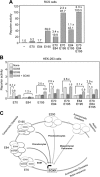
Two decades after the discovery that heterozygous mutations within and around SOX9 cause campomelic dysplasia, a generalized skeleton malformation syndrome, it is well established that SOX9 is a master transcription factor in chondrocytes. In contrast, the mechanisms whereby translocations in the --350/-50-kb region 5' of SOX9 cause severe disease and whereby SOX9 expression is specified in chondrocytes remain scarcely known. We here screen this upstream region and uncover multiple enhancers that activate Sox9-promoter transgenes in the SOX9 expression domain. Three of them are primarily active in chondrocytes. E250 (located at -250 kb) confines its activity to condensed prechondrocytes, E195 mainly targets proliferating chondrocytes, and E84 is potent in all differentiated chondrocytes. E84 and E195 synergize with E70, previously shown to be active in most Sox9-expressing somatic tissues, including cartilage. While SOX9 protein powerfully activates E70, it does not control E250. It requires its SOX5/SOX6 chondrogenic partners to robustly activate E195 and additional factors to activate E84. Altogether, these results indicate that SOX9 expression in chondrocytes relies on widely spread transcriptional modules whose synergistic and overlapping activities are driven by SOX9, SOX5/SOX6 and other factors. They help elucidate mechanisms underlying campomelic dysplasia and will likely help uncover other disease mechanisms.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Foster J.W., Dominguez-Steglich M.A., Guioli S., Kwok C., Weller P.A., Stevanovic M., Weissenbach J., Mansour S., Young I.D., Goodfellow P.N., et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. - PubMed
-
- Wagner T., Wirth J., Meyer J., Zabel B., Held M., Zimmer J., Pasantes J., Bricarelli F.D., Keutel J., Hustert E., et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. - PubMed
-
- Unger S., Scherer G., Superti-Furga A. GeneReviews. Seattle: University of Washington; 2013. Campomelic dysplasia. - PubMed
-
- Stolt C.C., Wegner M. SoxE function in vertebrate nervous system development. Int. J. Biochem. Cell Biol. 2010;42:437–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

