Niche signaling promotes stem cell survival in the Drosophila testis via the JAK-STAT target DIAP1
- PMID: 25941003
- PMCID: PMC4469572
- DOI: 10.1016/j.ydbio.2015.04.017
Niche signaling promotes stem cell survival in the Drosophila testis via the JAK-STAT target DIAP1
Abstract
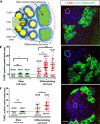
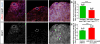
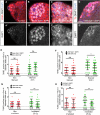
Tissue-specific stem cells are thought to resist environmental insults better than their differentiating progeny, but this resistance varies from one tissue to another, and the underlying mechanisms are not well-understood. Here, we use the Drosophila testis as a model system to study the regulation of cell death within an intact niche. This niche contains sperm-producing germline stem cells (GSCs) and accompanying somatic cyst stem cells (or CySCs). Although many signals are known to promote stem cell self-renewal in this tissue, including the highly conserved JAK-STAT pathway, the response of these stem cells to potential death-inducing signals, and factors promoting stem cell survival, have not been characterized. Here we find that both GSCs and CySCs resist cell death better than their differentiating progeny, under normal laboratory conditions and in response to potential death-inducing stimuli such as irradiation or starvation. To ask what might be promoting stem cell survival, we characterized the role of the anti-apoptotic gene Drosophila inhibitor of apoptosis 1 (diap1) in testis stem cells. DIAP1 protein is enriched in the GSCs and CySCs and is a JAK-STAT target. diap1 is necessary for survival of both GSCs and CySCs, and ectopic up-regulation of DIAP1 in somatic cyst cells is sufficient to non-autonomously rescue stress-induced cell death in adjacent differentiating germ cells (spermatogonia). Altogether, our results show that niche signals can promote stem cell survival by up-regulation of highly conserved anti-apoptotic proteins, and suggest that this strategy may underlie the ability of stem cells to resist death more generally.
Keywords: DIAP1; Drosophila spermatogenesis; JAK–STAT signaling; Stem cell niche.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Allan DJ, Harmon BV, Roberts SA. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif. 1992;25:241–250. - PubMed
-
- Blanpain C, Mohrin M, Sotiropoulou P. a, Passegué E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi:10.1016/j.stem.2010.12.012. - PubMed
-
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi:10.1126/science.1097676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

