Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33
- PMID: 25941360
- PMCID: PMC4466705
- DOI: 10.1073/pnas.1424236112
Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33
Abstract
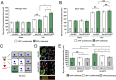
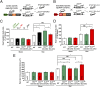
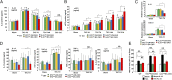
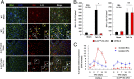
Hypertension increases the pressure load on the heart and is associated with a poorly understood chronic systemic inflammatory state. Interleukin 33 (IL-33) binds to membrane-bound ST2 (ST2L) and has antihypertrophic and antifibrotic effects in the myocardium. In contrast, soluble ST2 appears to act as a decoy receptor for IL-33, blocking myocardial and vascular benefits, and is a prognostic biomarker in patients with cardiovascular diseases. Here we report that a highly local intramyocardial IL-33/ST2 conversation regulates the heart's response to pressure overload. Either endothelial-specific deletion of IL33 or cardiomyocyte-specific deletion of ST2 exacerbated cardiac hypertrophy with pressure overload. Furthermore, pressure overload induced systemic circulating IL-33 as well as systemic circulating IL-13 and TGF-beta1; this was abolished by endothelial-specific deletion of IL33 but not by cardiomyocyte-specific deletion of IL33. Our study reveals that endothelial cell secretion of IL-33 is crucial for translating myocardial pressure overload into a selective systemic inflammatory response.
Keywords: cardiac hypertrophy; endothelial cells; inflammation; interleukin-33.
Conflict of interest statement
Conflict of interest statement: Brigham and Women’s Hospital holds patents on ST2, listing R.T.L. as inventor.
Figures

Comment in
-
Stressed hearts inflame the body (in a good way).Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7113-4. doi: 10.1073/pnas.1507821112. Epub 2015 May 21. Proc Natl Acad Sci U S A. 2015. PMID: 25997444 Free PMC article. No abstract available.
References
-
- Kearney PM, et al. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365(9455):217–223. - PubMed
-
- Shimpo M, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109(18):2186–2190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

