Organization and dynamics of the nonhomologous end-joining machinery during DNA double-strand break repair
- PMID: 25941401
- PMCID: PMC4443322
- DOI: 10.1073/pnas.1420115112
Organization and dynamics of the nonhomologous end-joining machinery during DNA double-strand break repair
Abstract
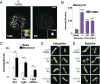
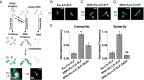
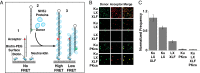
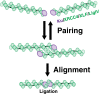
Nonhomologous end-joining (NHEJ) is a major repair pathway for DNA double-strand breaks (DSBs), involving synapsis and ligation of the broken strands. We describe the use of in vivo and in vitro single-molecule methods to define the organization and interaction of NHEJ repair proteins at DSB ends. Super-resolution fluorescence microscopy allowed the precise visualization of XRCC4, XLF, and DNA ligase IV filaments adjacent to DSBs, which bridge the broken chromosome and direct rejoining. We show, by single-molecule FRET analysis of the Ku/XRCC4/XLF/DNA ligase IV NHEJ ligation complex, that end-to-end synapsis involves a dynamic positioning of the two ends relative to one another. Our observations form the basis of a new model for NHEJ that describes the mechanism whereby filament-forming proteins bridge DNA DSBs in vivo. In this scheme, the filaments at either end of the DSB interact dynamically to achieve optimal configuration and end-to-end positioning and ligation.
Keywords: DNA repair; genomic integrity; nonhomologous end-joining; single-molecule FRET; super-resolution microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Weinfeld M, Lees-Miller SP. DNA double-strand break repair by non-homologous end joining and its clinical relevance. In: Kelley MR, editor. DNA Repair in Cancer Therapy: Molecular Targets and Clinical Applications. 2012. pp. 161–189.
-
- Lees-Miller SP, Meek K. Repair of DNA double-strand breaks by non-homologous end-joining. Biochimie. 2003;85(11):1161–1173. - PubMed
-
- Limp-Foster M, Kelley MR. DNA repair and gene therapy: Implications for translational uses. Environ Mol Mutagen. 2000;35(2):71–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

