Ff-nano, short functionalized nanorods derived from Ff (f1, fd, or M13) filamentous bacteriophage
- PMID: 25941520
- PMCID: PMC4403547
- DOI: 10.3389/fmicb.2015.00316
Ff-nano, short functionalized nanorods derived from Ff (f1, fd, or M13) filamentous bacteriophage
Abstract
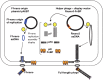
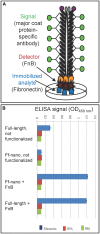
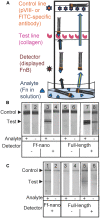
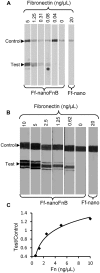
F-specific filamentous phage of Escherichia coli (Ff: f1, M13, or fd) are long thin filaments (860 nm × 6 nm). They have been a major workhorse in display technologies and bionanotechnology; however, some applications are limited by the high length-to-diameter ratio of Ff. Furthermore, use of functionalized Ff outside of laboratory containment is in part hampered by the fact that they are genetically modified viruses. We have now developed a system for production and purification of very short functionalized Ff-phage-derived nanorods, named Ff-nano, that are only 50 nm in length. In contrast to standard Ff-derived vectors that replicate in E. coli and contain antibiotic-resistance genes, Ff-nano are protein-DNA complexes that cannot replicate on their own and do not contain any coding sequences. These nanorods show an increased resistance to heating at 70(∘)C in 1% SDS in comparison to the full-length Ff phage of the same coat composition. We demonstrate that functionalized Ff-nano particles are suitable for application as detection particles in sensitive and quantitative "dipstick" lateral flow diagnostic assay for human plasma fibronectin.
Keywords: M13 phage; f1 phage; fd phage; fibronectin-binding protein; filamentous phage; lateral flow; nanorod; phage display.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

