Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization
- PMID: 25942370
- PMCID: PMC6272498
- DOI: 10.3390/molecules20057874
Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization
Abstract
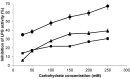
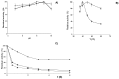
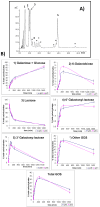
A novel β-galactosidase from Lactobacillus plantarum (LPG) was over-expressed in E. coli and purified via a single chromatographic step by using lowly activated IMAC (immobilized metal for affinity chromatography) supports. The pure enzyme exhibited a high hydrolytic activity of 491 IU/mL towards o-nitrophenyl β-D-galactopyranoside. This value was conserved in the presence of different divalent cations and was quite resistant to the inhibition effects of different carbohydrates. The pure multimeric enzyme was stabilized by multipoint and multisubunit covalent attachment on glyoxyl-agarose. The glyoxyl-LPG immobilized preparation was over 20-fold more stable than the soluble enzyme or the one-point CNBr-LPG immobilized preparation at 50 °C. This β-galactosidase was successfully used in the hydrolysis of lactose and lactulose and formation of different oligosaccharides was detected. High production of galacto-oligosaccharides (35%) and oligosaccharides derived from lactulose (30%) was found and, for the first time, a new oligosaccharide derived from lactulose, tentatively identified as 3'-galactosyl lactulose, has been described.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rodriguez A.P., Fernandez Leiro R., Cerdan M.E., Gonzalez M.I., Becerra-Fernandez M. Kluyveromyces lactis β-galactosidase crystallization using full-factorial experimental design. J. Mol. Catal. B.-Enzym. 2008;52:178–182. doi: 10.1016/j.molcatb.2007.11.013. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

