Epidermal Growth Factor Receptor Mutation Status in the Treatment of Non-small Cell Lung Cancer: Lessons Learned
- PMID: 25943319
- PMCID: PMC4614211
- DOI: 10.4143/crt.2014.362
Epidermal Growth Factor Receptor Mutation Status in the Treatment of Non-small Cell Lung Cancer: Lessons Learned
Abstract
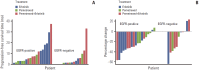
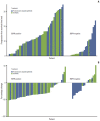
Advances in oncology research have led to identification of tumor-specific biomarkers, some of which are important predictive indicators and ideal targets for novel therapeutics. One such biomarker in non-small cell lung cancer (NSCLC) is the epidermal growth factor receptor (EGFR). Patients with NSCLC who harbor an activating EGFR mutation show a more favorable response to treatment with an EGFR inhibitor, such as gefitinib, erlotinib, or afatinib, than to chemotherapy. The prevalence of EGFR mutations in East Asian patients is higher than that in other populations, and in some clinical settings, patients have been treated with EGFR inhibitors based on clinicopathologic characteristics with no information on EGFR status. However, based on results from a series of studies in which East Asian patients with advanced non-squamous NSCLC were treated with EGFR inhibitors alone or in combination with standard chemotherapy, this may not be the best practice because EGFR mutation status was found to be a key predictor of outcome. Data from these studies highlight the necessity of EGFR testing in determining the most suitable treatment for patients with advanced or metastatic NSCLC.
Keywords: Biological markers; Epidermal growth factor receptor; Non-small-cell lung carcinoma.
Conflict of interest statement
This study was sponsored by Eli Lilly and Company, manufacturer/licensee of pemetrexed (Alimta). Eli Lilly and Company was involved in the design and preparation of the manuscript. RC, XW, and MO are employees of Eli Lilly and Company. RC and MO own shares in Eli Lilly Pty Ltd. DHL has received honoraria from Eli Lilly and Company as a member of an advisory board. VS has no relevant conflicts of interest to declare. Medical writing assistance was provided by Rose Boutros, PhD and Rebecca Lew, PhD, CMPP of ProScribe — Envision Pharma Group, and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2).
Figures
References
-
- Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–60. - PMC - PubMed
-
- Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

