Biocompatibility of single-walled carbon nanotube composites for bone regeneration
- PMID: 25943595
- PMCID: PMC4438669
- DOI: 10.1302/2046-3758.45.2000382
Biocompatibility of single-walled carbon nanotube composites for bone regeneration
Abstract
Objectives: The purpose of this study was to evaluate in vivo biocompatibility of novel single-walled carbon nanotubes (SWCNT)/poly(lactic-co-glycolic acid) (PLAGA) composites for applications in bone and tissue regeneration.
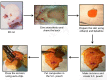
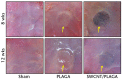
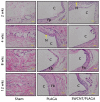
Methods: A total of 60 Sprague-Dawley rats (125 g to 149 g) were implanted subcutaneously with SWCNT/PLAGA composites (10 mg SWCNT and 1gm PLAGA 12 mm diameter two-dimensional disks), and at two, four, eight and 12 weeks post-implantation were compared with control (Sham) and PLAGA (five rats per group/point in time). Rats were observed for signs of morbidity, overt toxicity, weight gain and food consumption, while haematology, urinalysis and histopathology were completed when the animals were killed.
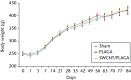
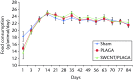
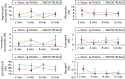
Results: No mortality and clinical signs were observed. All groups showed consistent weight gain, and the rate of gain for each group was similar. All groups exhibited a similar pattern for food consumption. No difference in urinalysis, haematology, and absolute and relative organ weight was observed. A mild to moderate increase in the summary toxicity (sumtox) score was observed for PLAGA and SWCNT/PLAGA implanted animals, whereas the control animals did not show any response. Both PLAGA and SWCNT/PLAGA showed a significantly higher sumtox score compared with the control group at all time intervals. However, there was no significant difference between PLAGA and SWCNT/PLAGA groups.
Conclusions: Our results demonstrate that SWCNT/PLAGA composites exhibited in vivo biocompatibility similar to the Food and Drug Administration approved biocompatible polymer, PLAGA, over a period of 12 weeks. These results showed potential of SWCNT/PLAGA composites for bone regeneration as the low percentage of SWCNT did not elicit a localised or general overt toxicity. Following the 12-week exposure, the material was considered to have an acceptable biocompatibility to warrant further long-term and more invasive in vivo studies. Cite this article: Bone Joint Res 2015;4:70-7.
Keywords: Biocompatibility; Bone regeneration; Bone tissue engineering; Carbon nanotubes; SWCNT/PLAGA composites.
©2015 The British Editorial Society of Bone & Joint Surgery.
Conflict of interest statement
Figures


References
-
- Borrelli J Jr, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res 2003;411:245–254. - PubMed
-
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury 2005;36(Suppl 3):S20–S27. - PubMed
-
- Korompilias AV, Lykissas MG, Soucacos PN, Kostas I, Beris AE. Vascularized free fibular bone graft in the management of congenital tibial pseudarthrosis. Microsurgery 2009;29:346–352. - PubMed
-
- Soucacos PN, Johnson EO, Babis G. An update on recent advances in bone regeneration. Injury 2008;39(suppl 2):S1–S4. - PubMed
-
- Petite H, Viateau V, Bensaïd W, et al. Tissue-engineered bone regeneration. Nat Biotechnol 2000;18:959–963. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

