The low-affinity complex of cytochrome c and its peroxidase
- PMID: 25944250
- PMCID: PMC4432590
- DOI: 10.1038/ncomms8073
The low-affinity complex of cytochrome c and its peroxidase
Abstract
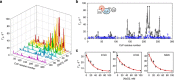
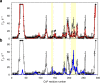
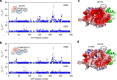
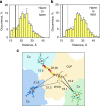
The complex of yeast cytochrome c peroxidase and cytochrome c is a paradigm of the biological electron transfer (ET). Building on seven decades of research, two different models have been proposed to explain its functional redox activity. One postulates that the intermolecular ET occurs only in the dominant, high-affinity protein-protein orientation, while the other posits formation of an additional, low-affinity complex, which is much more active than the dominant one. Unlike the high-affinity interaction-extensively studied by X-ray crystallography and NMR spectroscopy-until now the binding of cytochrome c to the low-affinity site has not been observed directly, but inferred mainly from kinetics experiments. Here we report the structure of this elusive, weak protein complex and show that it consists of a dominant, inactive bound species and an ensemble of minor, ET-competent protein-protein orientations, which summarily account for the experimentally determined value of the ET rate constant.
Figures

Similar articles
-
Structure and Function of Transient Encounters of Redox Proteins.Acc Chem Res. 2015 Dec 15;48(12):3036-43. doi: 10.1021/acs.accounts.5b00343. Epub 2015 Nov 25. Acc Chem Res. 2015. PMID: 26606503 Review.
-
Cytochrome c peroxidase binds two molecules of cytochrome c: evidence for a low-affinity, electron-transfer-active site on cytochrome c peroxidase.Biochemistry. 1993 Oct 12;32(40):10848-65. doi: 10.1021/bi00091a041. Biochemistry. 1993. PMID: 8399235
-
The complex of cytochrome c and cytochrome c peroxidase: the end of the road?Biochim Biophys Acta. 2011 Nov;1807(11):1482-503. doi: 10.1016/j.bbabio.2011.07.010. Epub 2011 Jul 28. Biochim Biophys Acta. 2011. PMID: 21820401 Review.
-
Solution structure and dynamics of the complex between cytochrome c and cytochrome c peroxidase determined by paramagnetic NMR.Proc Natl Acad Sci U S A. 2006 Dec 12;103(50):18945-50. doi: 10.1073/pnas.0603551103. Epub 2006 Dec 4. Proc Natl Acad Sci U S A. 2006. PMID: 17146057 Free PMC article.
-
Visualization of the encounter ensemble of the transient electron transfer complex of cytochrome c and cytochrome c peroxidase.J Am Chem Soc. 2010 Jan 13;132(1):241-7. doi: 10.1021/ja9064574. J Am Chem Soc. 2010. PMID: 19961227
Cited by
-
Constraints on the Radical Cation Center of Cytochrome c Peroxidase for Electron Transfer from Cytochrome c.Biochemistry. 2016 Aug 30;55(34):4807-22. doi: 10.1021/acs.biochem.6b00262. Epub 2016 Aug 17. Biochemistry. 2016. PMID: 27499202 Free PMC article.
-
Mechanistic insights into the chemistry of compound I formation in heme peroxidases: quantum chemical investigations of cytochrome c peroxidase.RSC Adv. 2022 May 23;12(24):15543-15554. doi: 10.1039/d2ra01073a. eCollection 2022 May 17. RSC Adv. 2022. PMID: 35685178 Free PMC article.
-
The Transient Complex of Cytochrome c and Cytochrome c Peroxidase: Insights into the Encounter Complex from Multifrequency EPR and NMR Spectroscopy.Chemphyschem. 2020 May 18;21(10):1060-1069. doi: 10.1002/cphc.201901160. Epub 2020 Apr 17. Chemphyschem. 2020. PMID: 32301564 Free PMC article.
-
Enhancing the population of the encounter complex affects protein complex formation efficiency.FEBS J. 2022 Jan;289(2):535-548. doi: 10.1111/febs.16159. Epub 2021 Sep 13. FEBS J. 2022. PMID: 34403572 Free PMC article.
-
The Charge Distribution on a Protein Surface Determines Whether Productive or Futile Encounter Complexes Are Formed.Biochemistry. 2021 Mar 16;60(10):747-755. doi: 10.1021/acs.biochem.1c00021. Epub 2021 Mar 1. Biochemistry. 2021. PMID: 33646750 Free PMC article.
References
-
- Erman J. E. & Vitello L. B. Yeast cytochrome c peroxidase: mechanistic studies via protein engineering. Biochim. Biophys. Acta 1597, 193–220 (2002). - PubMed
-
- Volkov A. N., Nicholls P. & Worrall J. A. R. The complex of cytochrome c and cytochrome c peroxidase: the end of the road? Biochim. Biophys. Acta 1807, 1482–1503 (2011). - PubMed
-
- Miller M.A. et al. Identifying the physiological electron transfer site of cytochrome c peroxidase by structure-based engineering. Biochemistry 35, 667–673 (1996). - PubMed
-
- Wang K. et al. Design of a ruthenium-cytochrome c derivative to measure electron transfer to the radical cation and oxyferryl heme in cytochrome c peroxidase. Biochemistry 35, 15107–15119 (1996). - PubMed
-
- Miller M. A. A complete mechanism for steady-state oxidation of yeast cytochrome c by yeast cytochrome c peroxidase. Biochemistry 35, 15791–15799 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

