Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta
- PMID: 25944380
- PMCID: PMC4795106
- DOI: 10.1038/ejhg.2015.81
Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta
Erratum in
-
Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta.Eur J Hum Genet. 2015 Aug;23(8):1112. doi: 10.1038/ejhg.2015.129. Eur J Hum Genet. 2015. PMID: 26177859 Free PMC article. No abstract available.
Abstract
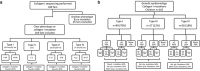
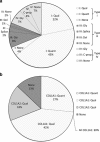
Osteogenesis imperfecta (OI) is a rare hereditary bone fragility disorder, caused by collagen I mutations in 90% of cases. There are no comprehensive genotype-phenotype studies on >100 families outside North America, and no population-based studies determining the genetic epidemiology of OI. Here, detailed clinical phenotypes were recorded, and the COL1A1 and COL1A2 genes were analyzed in 164 Swedish OI families (223 individuals). Averages for bone mineral density (BMD), height and yearly fracture rate were calculated and related to OI and mutation type. N-terminal helical mutations in both the α1- and α2-chains were associated with the absence of dentinogenesis imperfecta (P<0.0001 vs 0.0049), while only those in the α1-chain were associated with blue sclera (P=0.0110). Comparing glycine with serine substitutions, α1-alterations were associated with more severe phenotype (P=0.0031). Individuals with type I OI caused by qualitative vs quantitative mutations were shorter (P<0.0001), but did not differ considering fractures or BMD. The children in this cohort were estimated to represent >95% of the complete Swedish pediatric OI population. The prevalence of OI types I, III, and IV was 5.16, 0.89, and 1.35/100 000, respectively (7.40/100 000 overall), corresponding to what has been estimated but not unequivocally proven in any population. Collagen I mutation analysis was performed in the family of 97% of known cases, with causative mutations found in 87%. Qualitative mutations caused 32% of OI type I. The data reported here may be helpful to predict phenotype, and describes for the first time the genetic epidemiology in >95% of an entire OI population.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

