First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors
- PMID: 25944802
- PMCID: PMC4558321
- DOI: 10.1158/1078-0432.CCR-14-3321
First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors
Abstract
Purpose: Sacituzumab govitecan (IMMU-132) is an antibody-drug conjugate (ADC) targeting Trop-2, a surface glycoprotein expressed on many epithelial tumors, for delivery of SN-38, the active metabolite of irinotecan. This phase I trial evaluated this ADC as a potential therapeutic for pretreated patients with a variety of metastatic solid cancers.
Experimental design: Sacituzumab govitecan was administered on days 1 and 8 of 21-day cycles, with cycles repeated until dose-limiting toxicity or progression. Dose escalation followed a standard 3 + 3 scheme with 4 planned dose levels and dose delay or reduction allowed.
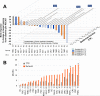
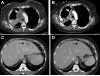
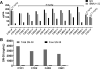
Results: Twenty-five patients (52-60 years old, 3 median prior chemotherapy regimens) were treated at dose levels of 8 (n = 7), 10 (n = 6), 12 (n = 9), and 18 (n = 3) mg/kg. Neutropenia was dose limiting, with 12 mg/kg the maximum tolerated dose for cycle 1, but too toxic with repeated cycles. Lower doses were acceptable for extended treatment with no treatment-related grade 4 toxicities and grade 3 toxicities limited to fatigue (n = 3), neutropenia (n = 2), diarrhea (n = 1), and leukopenia (n = 1). Using CT-based RECIST 1.1, two patients achieved partial responses (triple-negative breast cancer, colon cancer) and 16 others had stable disease as best response. Twelve patients maintained disease control with continued treatment for 16 to 36 weeks; 6 survived 15 to 20+ months. No preselection of patients based on tumor Trop-2 expression was done.
Conclusions: Sacituzumab govitecan had acceptable toxicity and encouraging therapeutic activity in patients with difficult-to-treat cancers. The 8 and 10 mg/kg doses were selected for phase II studies.
Trial registration: ClinicalTrials.gov NCT01631552.
©2015 American Association for Cancer Research.
Figures
References
-
- Younes A, Yasothan U, Kirkpatrick P. Brentuximab vedotin. Nat Rev Drug Discov. 2011;11:19–20. - PubMed
-
- Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. - PubMed
-
- Krop I, Winer EP. Trastuzumab emtansine: A novel antibody–drug conjugate for HER2-positive breast cancer. Clin Cancer Res. 2014;20:15–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

