Effect of Intensive Versus Standard Blood Glucose Control in Patients With Type 2 Diabetes Mellitus in Different Regions of the World: Systematic Review and Meta-analysis of Randomized Controlled Trials
- PMID: 25944874
- PMCID: PMC4599400
- DOI: 10.1161/JAHA.114.001577
Effect of Intensive Versus Standard Blood Glucose Control in Patients With Type 2 Diabetes Mellitus in Different Regions of the World: Systematic Review and Meta-analysis of Randomized Controlled Trials
Abstract
Background: Regional variation in type 2 diabetes mellitus care may affect outcomes in patients treated with intensive versus standard blood glucose control. We sought to evaluate these differences between North America and the rest of the world.
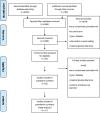
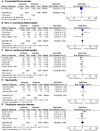
Methods and results: Databases were searched from their inception through December 2013. Randomized controlled trials comparing the effects of intensive therapy with standard therapy for macro- and microvascular complications in adults with type 2 diabetes mellitus were selected. We calculated summary odds ratios (ORs) and 95% CIs with the random-effects model. The analysis included 34 967 patients from 17 randomized controlled trials (7 in North America and 10 in the rest of the world). There were no significant differences between intensive and standard therapy groups for all-cause mortality (OR 1.03, 95% CI 0.93 to 1.13) and cardiovascular mortality (OR 1.09, 95% CI 0.90 to 1.32). For trials conducted in North America, intensive therapy compared with standard glycemic control resulted in significantly higher all-cause mortality (OR 1.21, 95% CI 1.05 to 1.40) and cardiovascular mortality (OR 1.41, 95% CI 1.05 to 1.90) than trials conducted in the rest of the world (all-cause mortality OR 0.93, 95% CI 0.85 to 1.03; interaction P=0.006; cardiovascular mortality OR 0.89, 95% CI, 0.79 to 1.00; interaction P=0.007). Analysis of individual macro- and microvascular outcomes revealed no significant regional differences; however, the risk of severe hypoglycemia was significantly higher in trials of intensive therapy in North America (OR 3.52, 95% CI 3.07 to 4.03) compared with the rest of the world (OR 1.45, 95% CI 0.85 to 2.47; interaction P=0.001).
Conclusion: Randomization to intensive glycemic control in type 2 diabetes mellitus patients was associated with increases in all-cause mortality, cardiovascular mortality, and severe hypoglycemia in North America compared with the rest of the world. Further investigation into the pathobiology or patient variability underlying these findings is warranted.
Keywords: cardiovascular mortality; diabetes mellitus; intensive glycemic control.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures






References
-
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. - PubMed
-
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. Erratum in: Lancet 1998;352:1558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

